Abstract
We investigated the interactions between liposome-encapsulated hemoglobin (Neo Red Cells: NRC) and human polymorphonuclear leukocytes as assessed by superoxide generation. NRC triggered superoxide generation from neutrophils in a dose-dependent manner. Empty liposomes also induced superoxide production of neutrophils. Superoxide generation of neutrophils induced by phorbol myristate acetate (PMA) was delayed but intensified both by NRC and empty liposomes. The intensity of superoxide generation induced by NRC was smaller than that by the empty liposomes. As NRC contained superoxide dismutase (SOD) that was copurified with hemoglobin from red blood cells and its activity remained, SOD contained in NRC may partially eliminate superoxide.
INTRODUCTION
Red blood cell (RBC) substitutes as artificial oxygen carriers have been developed to prevent transfusion-associated adverse effects such as viral transmission and to extend the storage periods of RBC components Citation[[1]]. Encapsulation of hemoglobin into liposomes is an advanced candidate for RBC substitutes compared to acellular hemoglobin Citation[[2]]. Anchoring poly(ethylene glycol) (PEG)-conjugated lipids into liposomes is the most promising approach to evade the reticuloendothelial system (RES) Citation[[3]], which is composed of the liver (Kupffer cells), the spleen, monocytes and macrophages. PEG modification also prevents complement activation in human blood Citation[[4]], while PEG-unmodified liposomes activate the complement system Citation[[5]]. Neo Red Cells (NRC; TERUMO Co., Tokyo, Japan), a liposome-encapsulated hemoglobin, have been developed and characterized by their physicochemical properties and oxygen carrying ability Citation[[6]]. However, it remains unclear whether NRC is tolerably biocompatible. We reported that NRC modified the function of monocytes, such as cytokine and superoxide production, and the expression of cell surface molecules, such as CD80 and CD54 Citation[7-8].
It is well known that neutrophils play an important role in host defense by generating superoxide to kill bacteria in response to phagocytosis Citation[9-10]. Superoxide generation by neutrophils is induced not only by phagocytosis but also by external stimuli such as phorbol myristate acetate (PMA) Citation[[11]], bacteria-derived chemoattractant peptide (N-formyl-methionyl-leucyl-phenylalanine) Citation[[12]], interleukin-8 Citation[[13]] and fatty acids Citation[[14]]. However, little is known about the effect of PEG-liposomes on the neutrophil-activation system. Superoxide generation by neutrophils is important for host defense. Therefore, we investigated the interaction between NRC and human polymorphonuclear leukocytes (PMN) in vitro by assessing superoxide generation.
MATERIALS AND METHODS
Preparation of Human Neutrophils
Human PMN was prepared from a buffy coat fraction of whole blood collected from voluntary donors in our blood center. Briefly, equal volumes of Hank's Balanced Salt Solution without Ca2+ and Mg2+ [HBSS(−)] (Gibco BRL, Rockville, MD, USA) and half volumes of 6% dextran (Nakarai, Kyoto, Japan) were added to the buffy coats, mixed well and left to stand for 30 min to allow the RBCs to settle. The upper layer was then applied onto a Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) in a 15 mL tube and centrifuged at 1,500 rpm for 30 min at room temperature. After hemolyzing RBC contaminated in precipitated cells by hypotonic shock with ice-cold H2O, the cells were washed with HBSS(−) twice and then suspended in HBSS containing Ca2+ and Mg2+ [HBSS(+)]. The cell (PMN) suspension was kept on ice and used as neutrophils.
Liposomes
NRC was used as a liposome-encapsulated hemoglobin () Citation[[6]]. Stroma-free hemoglobin solution used for NRC was prepared by hypotonic shock of human RBCs followed by centrifugation (18,700 × g for 30 min), 0.22 μm filtration and 300 kD ultrafiltration to remove the RBC membrane. The liposomes for encapsulation consisted of hydrogenated soy phosphatidylcholine:cholesterol:myristic acid (7:7:2) and were modified with PEG5000-phosphatidylethanolamine.
Table 1. Characteristics of NRC, Emply Liposomes, and Red Blood Cells (RBCs)
Empty liposomes were prepared in the same manner as NRC by TERUMO except that they were half the diameter, but did not contain any proteins ().
Measurement of Superoxide
Superoxide was detected by electron spin resonance (ESR) spectroscopy using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO; Sigma, St. Louis, MO, USA) as a spin trap reagent. Either NRC or empty liposomes were added to the PMN suspension (4 × 106 cells/reaction mixture) containing DMPO (final 53 mM) in a tube at time 0. PMA (Sigma) was added to the reaction mixture at the final concentration of 1 μM when required. The reaction mixture was then immediately transferred into an ESR cuvette and the ESR spectrum was measured at several time intervals at room temperature Citation[[15]]. In another experiment, superoxide was generated enzymatically using the hypoxanthine-xanthine oxidase (Sigma) reaction in the presence or absence of either NRC or empty liposomes. Typical results were shown in each figure, and we confirmed the reproducibility of each phenomenon in two or more experiments.
Measurement of SOD Activity and Hemoglobin Concentration
The activity of SOD was determined by a spectrophotometric assay Citation[[16]] according to the instructions of the kit (Bioxytech SOD-525; OXIS International Inc., Portland, OR, USA). As hemoglobin interferes with this assay, hemoglobin in the hemolysate (fresh RBC:H2O = 1:4) and NRC was extracted with 1.6 fold ice-cold ethanol/chloroform (5:3). Empty liposomes and H2O, as a control, were also treated in the same way. After the centrifugation, the aqueous upper phase was used for the measurement.
The hemoglobin concentration of the hemolysate was measured based on the cyanmethemoglobin method using Hemoglobin-Test Wako (Wako, Osaka, Japan).
RESULTS
Comparison of NRC and the Empty Liposomes
As stroma-free hemoglobin solution encapsulated in NRC is considered to contain not only hemoglobin but also other RBC-internal proteins such as SOD, we determined the SOD activity in NRC. As shown in , NRC contained SOD and its activity was comparable to that of fresh RBC.
Superoxide Generation from Neutrophils by Liposomes
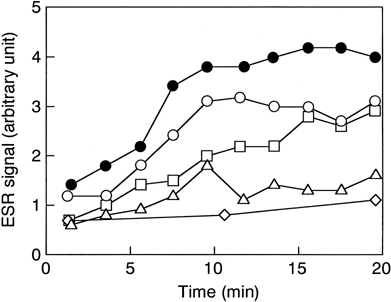
When neutrophils were mixed with either NRC or empty liposomes, an ESR signal was generated and gradually increased in the observation period (). The intensity of the ESR signal induced by NRC was smaller than that by the empty liposomes. The ESR spectra showed a typical DMPO-OOH pattern, the aduct formed by superoxide (data not shown). We then confirmed that the ESR signal disappeared following the addition of 50 mU of SOD ().
Figure 1. Superoxide generation from human neutrophils by NRC and empty liposomes. Liposomes were added at a final concentration of 8% (v/v) to PMN suspension containing DMPO. Open circles, NRC; closed circles, the empty liposomes; closed triangles, the empty liposomes + 50 mU of SOD.
Neither 50% (v/v) of NRC nor the empty liposomes enhanced the ESR signal which was generated enzymatically using the hypoxanthine-xanthine oxidase reaction, indicating that liposomes did not artificially potentiate ESR signals (data not shown). These results indicated that superoxide was generated from neutrophils by the interaction with NRC and the empty liposomes.
Superoxide generation from neutrophils appeared to be NRC-dose dependent (). A trace amount of superoxide was detected in the presence of 0.01% (v/v) NRC.
Effects of Liposomes on PMA-Induced Superoxide Generation from Neutrophils
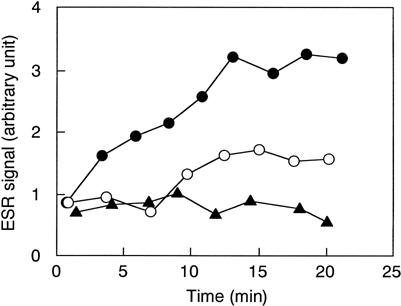
PMA-induced superoxide from neutrophils was more than 10-fold higher than that induced by NRC or the empty liposomes (). PMA-induced superoxide generation was delayed but intensified in the presence of NRC or the empty liposomes. The intensity of the ESR signal by NRC plus PMA was small compared to that by the empty liposomes plus PMA.
Figure 3. Effect of NRC and the empty liposomes on PMA-induced superoxide generation from human neutrophils. The liposomes were added at a final concentration of 10% (v/v) to PMN suspension containing DMPO, and then PMA was added to the reaction mixture. Squares, NRC; triangles, the empty liposomes; circles, PMA only.
DISCUSSION
PEG modification of liposomes was originally designed to evade the RES and to prolong the blood circulation period of liposomes. Goins et al. reported that PEG modification on liposomes prolonged blood circulation 1.6 times longer compared to unmodified liposomes (half-lifetime = 36.3 hr vs. 23.2 hr) Citation[[17]]. However, that study did not provide the information whether the PEG-liposomes interacted with leukocytes. We demonstrated here that PEG-liposomes (NRC) and empty liposomes interacted with neutrophils, resulting in superoxide generation ( and ). The mechanisms underlying the superoxide generation induced by the PEG-liposomes were not examined in this study. One possible mechanism is phagocytosis of PEG-liposomes by neutrophils.
The ESR signal induced by NRC was smaller than that by empty liposomes ( and ). It was reported that SOD incorporated in liposomes could scavenge superoxide generated by neutrophils Citation[[18]]. Because NRC maintained SOD activity at the same level as fresh human RBCs (), SOD in NRC partially eliminates superoxide generated from both PMA-induced and non-induced neutrophils.
We could not determine the amount of superoxide generated because of the difficulty of the quantification of superoxide by spin-trap ESR spectroscopy. To evaluate the clinical importance of liposome-induced superoxide generation, the amount of superoxide generated and its cytotoxicity should be addressed in further experiments. Recently, cross-linked hemoglobin conjugated with SOD and catalase, which are enzymes to scavenge reactive oxygen species, were reported to be effective in the prevention of intestinal ischemia-reperfusion injury Citation[[19]], hemoglobin damage and liposomal membrane peroxidation Citation[[20]]. Therefore, the characteristics of NRC, which contains SOD, will be beneficial to prevent organs and artificial oxygen carriers itself from oxidative stress Citation[[21]].
ACKNOWLEDGMENTS
Neo Red Cells and empty liposomes were provided by R&D Center, TERUMO co., Kanagawa, Japan.
REFERENCES
- Chang T M. Modified hemoglobin blood substitutes: present status and future perspectives. Biotechnol Annu Rev 1998; 4: 75–112
- Rudolph A S. Encapsulated hemoglobin: current issues and future goals. Artif Cells Blood Substit Immobil Biotechnol 1994; 22: 347–360
- Maruyama K. Prolonged circulation time in vivo of large unilamellar liposomes composed of distearoyl phosphatidylcholine and cholesterol containing amphipathic poly(ethylene glycol). Biochim Biophys Acta 1992; 1128: 44–49
- Bradley A J, Devine D V, Ansell S M, Janzen J, Brooks D E. Inhibition of liposome-induced complement activation by incorporated poly(ethylene glycol)-lipids. Arch Biochem Biophys 1998; 357: 185–194
- Szebeni J, Wassef N M, Hartman K R, Rudolph A S, Alving C R. Complement activation in vitro by the red cell substitute, liposome-encapsulated hemoglobin: mechanism of activation and inhibition by soluble complement receptor type 1. Transfusion 1997; 37: 150–159
- Ogata Y, Goto H, Kimura T, Fukui H. Development of Neo Red Cells (NRC) with the enzymatic reduction system of methemoglobin. Artif Cells Blood Substit Immobil Biotechnol 1997; 25: 417–427
- Niwa K, Ikebuchi K, Fujihara M, Abe H, Wakamoto S, Ito T, Yamaguchi M, Sekiguchi S. Inflammatory cytokine production in whole blood modified by liposome-encapsulated hemoglobin. Artif Cells Blood Substit Immobil Biotechnol 1998
- Fujihara M, Ikebuchi K, Yamaguchi M, Abe H, Niwa K, Sekiguchi S. Effects of liposome-encapsulated hemoglobin on phorbol ester-induced superoxide production and expression of costimulatory molecules by monocytes in vitro. Artif Cells Blood Substit Immobil Biotechnol 1998
- Klebanoff S J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem 1974; 249: 3724–3728
- Forman H J, Thomas M J. Oxidant production and bactericidal activity of phagocytes. Ann Rev Physiol 1986; 48: 669–680
- DeChatelet L R, Shirley P S, Johnston R B. Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood 1976; 47: 545–554
- Boxer L A, Yoder M, Bonsib S, Schmidt M, Ho P, Jersild R, Baehner R L. Effects of a chemotactic factor, N-formylmethionyl peptide, on adherence, superoxide anion generation, phagocytosis, and microtubule assembly of human polymorphonuclear leukocytes. J Lab Clin Med 1979; 93: 506–514
- Yuo A, Kitagawa S, Kasahara T, Matsushima K, Saito M, Takaku F. Stimulation and priming of human neutrophils by interleukin-8: cooperation with tumor necrosis factor and colony-stimulating factors. Blood 1991; 78: 2708–2714
- Tanaka T, Kanegasaki S, Makino R, Iizuka T, Ishimura Y. Saturated and trans-unsaturated fatty acids elicit high levels of superoxide generation in intact and cell-free preparations of neutrophils. Biochem Biophys Res Commun 1987; 144: 606–612
- Kuwabara M, Yamamoto T, Inanami O, Sato F. Mechanism of photosensitization by pheophorbide a studied by photohemolysis of erythrocytes and electron spin resonance spectroscopy. Photochem Photobiol 1989; 49: 37–41
- Nebot C, Moutet M, Huet P, Xu J Z, Yadan J C, Chaudiere J. Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal Biochem 1993; 214: 442–451
- Goins B, Phillips W T, Klipper R. Blood-pool imaging using technetium-99m-labeled liposomes. J Nucl Med 1996; 37: 1374–1379
- Somiya K, Niwa Y, Michelson A M. Effects of liposomal superoxide dismutase on human neutrophil activity. Free Radic Res Commun 1986; 1: 329–337
- Razack S, D'Agnillo F, Chang T M. Crosslinked hemoglobin-superoxide dismutase-catalase scavenges free radicals in a rat model of intestinal ischemia-reperfusion injury. Artif Cells Blood Substit Immobil Biotechnol 1997; 25: 181–192
- D'Agnillo F, Chang T. Absence of hemoprotein-associated free radical events following oxidant challenge of crosslinked hemoglobin-superoxide dismutase catalase. Free Radical Biol Med 1998; 24: 906–912
- Chang T M, D'Agnillo F, Yu W P, Razack S. Two future generations of blood substitutes based on polyhemoglobin-SOD-catalase and nanoencapsulation. Adv Drug Deliv Rev 2000; 40: 213–218