Abstract
The development of new therapeutic strategies and innovative biomaterials for the muscoloskeletal system, stresses the need for researchers to have reliable, easy, less time-consuming and ethical experimental models. The aim of the present study was to characterise an in vitro model of cultured rat femora and test the possibility of using this model in dynamic studies on bone healing. 24 femurs were explanted after 12 rats were killed for other experimental protocols. A standard bone defect was created in the distal femoral condyles and femurs were cultured in GBJb medium. Arginine and lysine were administered daily in the Arg-Lys group. The other femurs were left untreated (Control group). At 1, 7, 14 and 21 days, alkaline phosphatase activity, nitric oxide and calcium were measured on the supernatant. At 21 days, femurs were embedded in polymethylmethacrylate for histomorphometry and microhardness evaluation of the newly formed bone.
The current results showed that it was possible to study bone healing in vitro by using cultured bones from adult animals. A process for bone healing was observed also in untreated bones. Moreover, the structural analysis of the cultured bone showed that it had characteristics similar to those of the femurs, when they were embedded in resin immediately after animal sacrifice. The effect of Arg and Lys confirmed data of a previous study, where a faster healing of bone defect and fracture was observed in rabbits after Arg and Lys administration.
INTRODUCTION
The tremendous progress of the research and applied technologies in medicine, creates the need for easy, less time-consuming and reproducible methods, to test new drugs, physical therapies and biomaterials.
When focusing the attention on the muscoloskeletal research, we observe the presence of clinical situations still unsolved, such as pseudoarthrosis, large bone defects, cartilage lesions, osteoporosis, osteoarthritis, aseptic loosening of implants and prostheses, which are nothing but some of the pathologies representing a big problem for clinicians and patients Citation[[1]]. Researchers continuously develop or suggest new strategies that may be pre-clinically investigated with experimental models. Following ethical and legal principles, the current research methodology includes two main steps, i.e. tests on cell cultures and animal studies. In vitro studies based on bone tissue cultures may represent an appropriate bridge between cell cultures and in vivo animal researches, to improve the knowledge on new therapies or materials before animal testing. As far as we know, over the last years, bone tissue cultures were not extensively used in comparison with osteoblasts and staminal cell cultures, and were mainly selected by researchers for studies on bone metabolism Citation[2-9]. Moreover, many studies based on bone tissue cultures used fetal or embryonic bone segments Citation[3-4], Citation[7-9], that are known to be useful for basic research, but provide results which should be carefully transferred into clinical practice, these tissues being in a particular situation in terms of growth and potentialities.
In the present preliminary paper, a method based on bone tissue culture in adult rats was characterised in basal conditions by means of alkaline phosphatase activity (ALP), nitric oxide (NO) and calcium (Ca) dosage in the supernatant. Moreover, the suitability of the model for dynamic studies on bone healing was comparatively evaluated, by creating a bone defect and studying its rate of healing by histomorphometry and microhardness evaluation after administering essential amino acids (L-arginine and L-lysine). In our previous experiences, this therapy had proven to be effective in stimulating bone cells and bone healing in osteoblast cultures and rabbits, respectively Citation[10-11].
MATERIALS AND METHODS
Bone Tissue Culture
Twenty-four femora were dissected from 12 adult Sprague Dawley rats (360 ± 30 g). Animals were used in compliance with both the European and Italian Laws on animal experimentation, and the Animal Welfare Assurance No A5424-01 by the National Institute of Health (NIH-Rockville Maryland, USA).
Under aseptic conditions, femora were explanted and soft tissues were dissected preserving the periosteum. Then, femora were washed in 37°C phosphate buffered saline solution, and a bone defect of standard size (3.80 mm2) was created in the distal femoral condyle with a stainless-steel drill. Afterwards, femora were maintained in BGJb culture medium (Fitton-Jackson modification, Sigma, St.Louis, MO, USA), supplemented with 20% fetal calf serum (Boehringer Mannheim, Germany), penicillin 100 U/ml and streptomycin 100 mg/ml, (Sigma, St.Louis, MO, USA), β-glycero-phosphate 10mM (Sigma, St.Louis, MO, USA) and ascorbic acid 50μg/ml (Sigma, St.Louis, MO, USA) (BGJb plus); finally, they were incubated at 37°C in 95%air/5%CO2 atmosphere.
Over a period of three weeks, twelve femora cultivated in the BGJb plus medium, were treated daily with L-arginine (Arg) (Sigma, St.Louis, MO, USA) and L-lysine (Lys) (Sigma, St.Louis, MO, USA) at a concentration of 0.625mg/ml/day and 0.587mg/ml/day, respectively (Arg-Lys Group) The dosages were those recommended for clinical use and administered in our previous in vitro and studies Citation[10-11]. The other 12 femora, cultured in the same medium and conditions but without amino acid treatment, were used as Control Group.
Culture medium was changed twice a week and no bacterial or fungal contamination was found during experiment.
Biochemical and Histomorphometric Analysis
Supernatant of all cultures was collected at 1(basal), 7, 14, and 21 days, and the following parameters were calculated: alkaline phosphatase activity (ALP Sigma kit, St.Louis, MO, USA), nitric oxide (NO colorimetric assay, Sigma, St.Louis, MO, USA) and calcium (Ca Sigma kit, St.Louis, MO, USA).
At the last scheduled experimental time, femora from both Arg-Lys and control groups were fixed in 4% paraformaldehyde, dehydrated in alcohol grading scale, infiltrated in methylmethacrylate and embedded in polymethylmethacrylate. Samples were sectioned (40μ thickness) with a diamond saw microtome (Leica 1600, Ernst Leitz, Wetzlar, Germany), and then stained with Fast Green. A computerised system (Kontron S300, v.2, Kontron Elektronik, Munchen, Germany) was used to analyse healing of the defect. The healing percentage of the defect area was calculated from this formula:Resin-embedded specimens, ground and polished, were used to measure the level of bone hardness by means of an indentation test (Microhardness VMHT 30, Leica, Wien, Austria). Microhardness measurements were made with a Vickers indenter (four-sided pyramid with square base and an apex angle between opposite sides of 136°15′) applied to the cancellous bone at a load of 0.05 kgf and dwell time of 5 sec. The Vickers hardness degree (HV) was calculated by dividing the indentation force by the surface of the imprint (4 pyramid surfaces) observed at the microscope. The resulting formula was
where F is the weight applied to the pyramid expressed in Kg, β is half of the pyramid angle, and d is the average diagonal length of the imprint expressed in μm. The average value per sample was calculated on a mean of 10 for each examined area, both on the newly formed bone inside the defect and at 1000 μm from it (normal pre-existing bone); by allowing a minimum distance of about 3d between the imprints, also their mutual influence was avoided.
Statistical Analysis
Statistical analysis was performed by using SPSS v.7.5 software (SPSS/PC Inc., IL, USA). Data are given as mean ± SD at a significant level of p < 0.05. After having verified normal distribution and homogeneity of variances, the associations between variables yielding Pearson's correlation coefficients were detected. The ANOVA test and post hoc multiple comparison tests were used to compare data among groups.
RESULTS
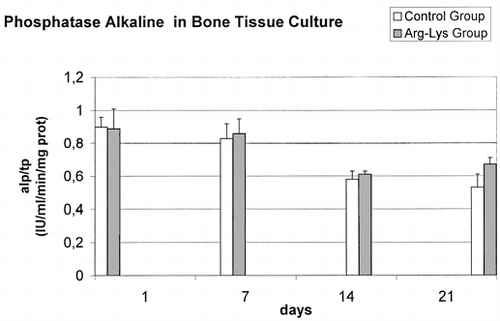
Data had homogeneity of variance and were normal distributed. No correlations were found among considered variables. Adjusted for total proteins ALP dosage, Ca content and NO production showed similar medium basal value in both groups without any significant difference. In controls, subsequent evaluations at 7, 14 and 21 days highlighted () a constant decrease of the ALP/TP values (F = 48.01, p<0.0005), thus revealing a loss of the metabolic activity of bone during the culture. Particularly, significant (p<0.0005) decreases in ALP/PT activity were observed between 1 and 14 days (Δ = −0.31 IU/ml), 1 and 21 days (Δ = −0.37 IU/ml), 7 and 14 days (Δ = −0.24 IU/ml), and between 7 and 21 days (Δ = −0.30 IU/ml) data. Also in the Arg-Lys treated groups a ALP/TP decrease (F = 17.07, p<0.0005) was noticed but itwas less marked than in the control group: significant decreases in ALP/PT activity were observed between 1 and 14 days (Δ = −0.28 IU/ml, p<0.0005), 1 and 21 days (Δ = −0.22 IU/ml, p<0.005), 7 and 14 days (Δ = −0.25 IU/ml, p<0.0005), and finally between 7 and 21 days (Δ = −0.20 IU/ml, p<0.01) data. In addition, ALP/TP data of Arg-Lys group was significant higher than controls at 21 days (t = −3.033 p<0.05).
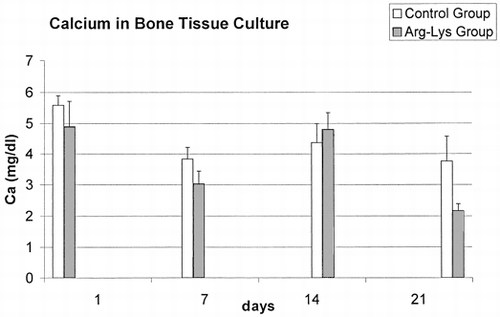
The dosage of Ca in the culture medium is reported in . Significant decreases in Ca content of 21% and 42% between groups were observed at 7 days (t=3.793, p<0.005) and 21 days (t=3.088, p < 0.05), respectively. The lowest values observed in the supernatant of the Arg-Lys group at 21 days, could depend on the greater amount of Ca used by cells for the mineralization of newly formed bone. ANOVA highlighted also significant variations in Ca content for both groups (Control: F=4.39, p<0.05; Treated: F=36.91, p<0.0005) during the time. In fact, significant (p < 0.05) decreases in Ca content were observed between 1 and 7 days (Δ = −1.49 mg/dl, p<0.05), and 1 and 21 days (Δ = −1.56 mg/dl, p<0.05) data of Control group, while decreases between 1 and 7 days (Δ = −1.87 mg/dl, p<0.0005), 1 and 21 days (Δ = −2.73 mg/dl, p<0.0005), and 14 days and 21 days (Δ = −2.64 mg/dl, p<0.0005), and finally an increase between 7 days and 14 days (Δ = 1.78 mg/dl, p<0.0005) data of Arg-Lys group.
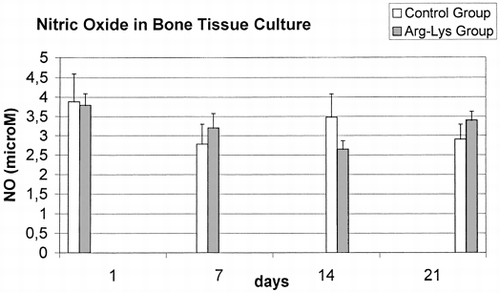
In reference to NO results (), a significantly constant decrease was found from 1 days to 21 days results in Control group F=4.18, p<0.05), but not in Arg-Lys group (F=0.62, ns). NO data showed also a significant difference between control and Arg-Lys groups at 21 days (t= −2.66, p<0.05).
Histomorphometric analysis of the defect is reported in ; after 21 days, the surface of the defect area in the Arg-Lys group was smaller than in the control group, with a significant difference in the healing rate (26.3% in Arg-Lys vs 7.9 % in controls).
Table 1. Histomorphometric Measurements of Bone Healing in Defect Treated with Arg-Lys and Control Group
Microhardness measurements in the newly formed bone inside the defect area provided data similar to those obtained in the pre-existing bone (): no significant differences were observed between Arg-Lys and control groups, thus demonstrating that the quality of the newly formed bone was good also in the untreated defects. Values obtained for trabecular bone hardness in cultured femora and in femurs explanted from animals, were also compared (data not shown) after methacrylate-embedding, and found to be superposable, thus proving the reliability of the in vitro tissue culture model which could preserve bone characteristics.
Table 2. Microhardness Evaluation (Vickers Degree) of Newly Mineralized Bone in the Healing Area of the Femur Defect Both in Treated and in Control Groups. Data Are Compared with Values of the Pre-existing Bone (Measurements Taken at Least at 1 mm from the Defect)
DISCUSSION
The increasing number of new and promising therapies for muscoloskeletal pathologies requires continuous testing and involves both the development of many experimental methodologies and the adoption of different experimental in vitro and in vivo models. At the same time, complex ethical, political, legal and economic issues impose restrictions on the use of animals, which can be employed only for therapies and materials that have already demonstrated their efficacy and safety in vitro.
The use of cell cultures from different tissues is recognised to be of great importance in any research, because of the advantages in examining reactions in standard conditions without the involvement of the numerous factors characterising the in vivo situation. However, it was sometimes observed that therapies and methods are often overestimated when tested in cell cultures, because of the increased cell responsiveness. After working on the effect of electromagnetic stimulation on cells, Spadaro suggested that experiments on cell cultures could present an altered sensitivity to applied stimuli because of the absence of matrix environment, and he stated that intermediate organ cultures might represent a useful compromise Citation[[12]].
Sun J-S et al used a bone tissue culture from adult rats maintained for 1 and 2 weeks to study bone fracture healing after ultrasound stimulation Citation[[13]], and following their example we performed an in vitro study with cultured adult rat femurs aiming to
prepare a preliminary characterisation of the model, also in case of longer culture time
test the possibility of performing in vitro dynamic studies on bone defect healing
improve our previous data on the effect of an amino acidic therapy on bone defect healing Citation[10-11].
Our results showed that it was possible to study bone healing in vitro, by using bones cultured from adult animals. A process of bone healing was observed also in untreated bones; moreover, the structural analysis of the newly formed bone showed that it had characteristics similar to those of the femurs, when they were embedded in resin immediately after animal sacrifice.
Concerning the effect of Arg and Lys, our data support the conclusions of a previous study, where faster healing of bone defect and fracture was observed in rabbits after Arg and Lys administration Citation[10-11].
In conclusion, we think that bone tissue cultures may be more extensively used by researchers, being easy, less time-consuming and reliable. In addition, their use avoids unnecessary sacrifice of additional animals, since it is possible to use bones from animals sacrificed at the end of other experimental protocols. Furthermore, they allow to use types of bones affected with different pathologies by means of standard experimental animal models (i.e. osteoporosis), and also to perform biomechanical investigations on the newly formed bone, which have proven to be more reliable than those carried out in cell culture systems.
ACKNOWLEDGMENTS
Financial support of this research was given by the Rizzoli Orthopaedic Institute, “ricerca corrente”. Authors would like to thank C.Dalfiume, P.DiDenia, N.Corrado, F.Rambaldi, and P.Nini, Experimental Surgery Department, Rizzoli Orthopaedic Institute, for their assistance.
REFERENCES
- Karachalios T, Lyritis G P. New horizons in applied muscoloskeletal research. Calcif Tissue Int 2000; 66(3)165–167
- Gao Y H, Yamaguchi M. Anabolic effect of daidzein on cortical bone in tissue culture: comparison with genistein effect. Mol Cell Biochem 1999; 194(1–2)93–97
- Tsuzuike N, Segawa Y, Tagashira E, Yamaguchi M. Beta-alanyl-L-histidinato zinc enhances various bone-regulating factors' effects on bone alkaline phosphatase activity in tissue culture. Pharmacology 1993; 47(1)66–72
- Yamaguchi M, Kishi S. Comparison of the effect of beta-alanyl-L-histidinato zinc and its zinc-chelating ligand on bone metabolism in tissue culture. Biol Pharm Bull 1994; 17(4)522–526
- Athanassiades A, Anastassiades T P. A coupled subchondral bone-articular cartilage tissue culture system for the study of cartilage proteoglycan metabolism. In Vitro Cell Dev Biol Anim 1994; 30A(8)504–511
- Yamaguchi M, Gao Y H. Inhibitory effect of genistein on bone resorption in tissue culture. Biochem Pharmacol 1998; 55(1)71–6
- Tsuda M, Kitazaki T, Ito T, Fujita T. The effect of ipriflavone (TC-80) on bone resorption in tissue culture. J Bone Miner Res 1986; 1(2)207–211
- Yamaguchi M, Uto M, Matsui R. Stimulatory effect of calcitonin on bone in tissue culture. J Pharmacobiodyn 1989; 12(11)708–715
- Nefussi J R, Baron R. PGE2 stimulates both resorption and formation of bone in vitro: differential responses of the periosteum and the endosteum in fetal rat long bone cultures. Anat Rec 1985; 211(1)9–16
- Fini M, Nicoli Aldini N, Canè V, Zaffe D, Giavaresi G, Rocca M, Guzzardella G A, Giardino R. Effects of essential aminoacids and lactose on bony fractures and defects in rabbits : a preliminary histomorphometric study. Arch Orthop Trauma Surg 1999; 119: 39–45
- Fini M, Torricelli P, Giavaresi G, Carpi A, Nicolini A, Giardino R. Effect of L-lysine and L-arginine on primary osteoblast cultures from normal and osteopenic rats, In Press Biomedicine Biotherapy 2001
- Spadaro J A. Mechanical and electrical interactions in bone remodeling. Bioelectromagnetics 1997; 18: 193–202
- Sun J-S, Tsuang Y H, Lin F H, Liu H C, Tsai C Z, Chang W HS. Bone defect healing enhanced by ultrasound stimulation: an in vitro tissue culture model. J Biomed Mater Res 1999; 46: 253–261