Abstract
Neoglycoconjugates prepared by synthesis of oxidized mannans from Saccharomyces cerevisiae with bovine serum albumin were studied for interaction with Concanavalin A. The mannan-bovine serum albumin neoglycoproteins, different in degree of mannan oxidation used for synthesis and its content in conjugates were prepared. The interaction of these glycoconjugates with Concanavalin A by using precipitation method was investigated. The conjugates prepared at high weight ratio mannan: protein (4:1) involved 47–65 % of saccharides and formed by precipitation with Concanavalin A aggregates with low content of protein. The obtained results showed that conjugates with lower content of mannan (up to 30 %) are more efficacious for their aggregation with Concanavalin A than the conjugates with high content of mannan.
INTRODUCTION
The polysaccharides and glycoproteins containing the specific mono-, oligoor polysaccharide part possess the affinity to lectins. In biological systems, such affinities and their specificity are critical components of cell-cell recognition. From the precipitation studies on a large number of polysaccharides, Goldstein and co-workers suggested that all branched polysaccharides having multiple, terminal (nonreducing) α-glucopyranosyl, α-mannopyranosyl or fructofuranosyl groups would precipitate with lectin Concanavalin A (Con A). Citation[1-2]. It was found out, that the polymer carrying multiple specific mannose residues exhibited a multiply higher affinity for Con A than methyl-α-D-mannoside—the most interacting monosaccharide Citation[[3]]. The interaction of Con A with α-mannans and phosphomannans isolated from a variety of microorganisms was described Citation[[4]]. Yeast mannans are extensively branched polymers and the presence of extended sequences of α-D-(1 →2) linked mannose residues in many microbial mannans provides sites for interaction with Con A. On the other side a synthetic linear α-D-(1 →3) mannan did not precipitate with Con A Citation[[5]]. It shows the importance of glycosidic moiety shape on interaction with lectins.
The use of lectins as a tool for structural analysis, isolation and resolution of glycoproteins is the most important development in lectin biochemistry Citation[[6]]. Con A as well as many other lectins were widely used also in the affinity purification of a variety of glycoenzymes Citation[7-10] and nearly exclusively in the immobilization of glycoenzymes on a variety of supports Citation[11-12]. The positive effect of multivalent binding on the specificity of protein-carbohydrate interactions was confirmed at several synthesized carbohydrate-bearing polymers tested for their ability to bind to lectins Citation[13-15]. Since glycoconjugates have been shown to interact with many biological systems a great deal of attention has been focused on the development of polymer-glycosidic ligands Citation[[16]]. In priciple, a glycosidic ligand should retain its selective interaction after attachment to a polymeric material, despite some limiting factors arising from the heterogeneity introduced by the polymer. Therefore a large variety of glycopolymers have been designed and their behaviour toward different carbohydrate-binding proteins have been investigated Citation[17-18].
The present paper deals with the study of interaction of synthetic glycoproteins, prepared from mannan Saccharomyces cerevisiae and bovine serum albumin (BSA), with Con A by using precipitation method. To examine the effect of degree of degradation of bound mannans and their amount in neoglycoconjugates, on the affinity of mannan-BSA conjugates to Con A, were synthesized various glycoconjugates and tested them for their precipitation with Con A. This method will help to find the optimum type of oxidised mannan and its content in conjugates with proteins (enzymes) for formation of aggregates with Con A as well as for its immobilization on Con A sorbents.
MATERIALS AND METHODS
Materials
The mannan used (average molecular weight ≈ 46 000 kDa), isolated from Saccharomyces cerevisiae (mannan SC), was kindly provided by Dr.Šandula Citation[[19]]. Bovine serum albumin (BSA) was purchased from IMUNA, Šarišské Michal' any, Slovak Republic, sodium cyanoborohydride (NaCNBH3) was obtained from Fluka Chemie AG, Buchs, Switzerland, natrium borohydride (NaBH4) and sodium periodate (NaIO4) used were the products of Lachema, Brno, Czech Republic. Lectin Concanavalin A (Con A) was obtained form Lectinola, Prague, Czech Republic. Methyl-α-D-mannopyranoside (α-MMP) was purchased from Fluka Chemie AG, Buchs, Germany.
Preparation of Mannan Conjugates with BSA
Oxidation of Mannan
the mannan dialdehyde were prepared by oxidation with sodium periodate (0.05 M-aqueous solution) at molar ratio NaIO4/mannan = 10, 25 and 50. The reaction condition were: the stirring in the dark at 4°C during 1 h. The reaction was stopped by ethylene glycol and the oxidised mannans were dialyzed against water and afterwards lyophilized. 3 samples of oxidised mannan were obtained: M1 (11 aldehyde groups per mannan molecule), M2 (20 aldehyde groups per mannan molecule) and M3 (28 aldehyde groups per mannan molecule).
Conjugation Mannan with BSA
to the solutions of BSA in 0.05 M phosphate buffer pH 7 (4 ml, c = 10 mg.ml1) was added a solution of mannan dialdehyde M1, M2 or M3 (c = 10 mg.ml1 of the same buffer) of various volume. Three different reaction ratios of mannan to BSA(1:1; 1.5:1 and 4:1) were used. To each mixture was added NaCNBH3 (2.5 ml, c = 10 mg. ml1) and was stirred at room temperature. The reaction was stopped after 24 h by addition of NaBH4 solution (c = 5 mg.ml1) in 0.05-M borate buffer pH 9.5. The molar ratio of NaBH4 to determined aldehyde groups ca 50:1 was used to reduce the remaining aldehyde groups. The reduction was performed at room temperature and at stirring for 6 h. The products were adjusted with 4 M- HCl to pH 6–7 and dialyzed against H2O. Unreacted and degraded mannans were removed by ultrafiltration through membrane YM 30 (Amicon Inc. Beverly, USA) and the resulted water solutions of conjugates were lyophilized. The content of saccharide Citation[[20]] and proteins Citation[[21]] in lyophilizates were determined. The content of nitrogen was determined by elemental analysis of conjugates (EA 1108 CHN-O analyzer, Milan, Italy).
Determination of MolecularWeight of Mannans and Conjugates
Mw of the original, oxidized mannan (after their reduction by using NaBH4) as well as conjugates mannan-BSA samples were determined by HPSEC method. HPLC system (Shimadzu, Wien, Austria) was composed of high pressure pump LC-10AD, a membrane degasser GT-104, a injector Rheodyne 77251, differential refractometer RID-6A and UV VIS detector SPD-10AV. The system was calibrated with a set of pullulans with the Mw =5 – 100 kDa (Macherey-Nagel, Düren, Germany). The solutions of calibrating pullulans as well as of tested mannans (c = 1 mg.ml1 of water) were applied on a HEMA BIO 100 column (8 × 250 mm, 10 μm sorbent of particle size) obtained from Tessek, Prague, Czech Republic and eluted with 0.02 M-phosphate buffer pH 7.2 at flow rate 0.4 ml.min1. Mannan-BSA conjugates were run through a tandem of two columns (HEMA-BIO 100 followed by HEMA-BIO 1000, both of dimensions 8 × 250 mm and particles size 10 μm). The system was calibrated with a set of glycoproteins Mw = 45 – 450 kDa. Flow rates 0.5 ml.min1. The mobile phase was 0.02M phosphate buffer of pH 7.2 and the volume of injected sample was 0.02 ml in both systems. The differential refractometer served as the polysaccharide concentration detector and by using UV detector the proteins were was determined. The elution profile was recorded by Class-VP-chromatography software.
Quantitative Precipitation Assay
Precipitation of mannan SC as well as mannan-BSA conjugates with Con A was assayed in phosphate buffer pH 7 containing 0.05 mol.L1 sodium phosphate, 0.1 mol.L1 NaCl, 0.1 mmol.L1 CaCl2 and 0.1 mmol.L1 MnCl2 × 4 H2O. The total volume of precipitation tests was 1 ml containing 230 μg.ml1 of Con A and various concentration of original mannan (within the range from 20 to 190 μg.ml1) or conjugate (within the range from 28 to 473 μg.ml1). The samples were shaken at 25°C for 2 h. The precipitates were separated in a microcentrifuge MPW-310 (Mechanika Precyzyna, Warszawa, Poland) at 10 000 min1 (8 400 g) during 10 min, washed twice with 1 M-NaCl, centrifuged and subsequently dissolved in 0.6 ml of 0.05 M-phosphate buffer adjusted to pH 10.5 with 1M-NaOH. These solutions were analyzed for saccharides Citation[[20]] and proteins Citation[[21]].
RESULTS AND DISCUSSION
The conjugates mannan-BSA were prepared, where 3 types of oxidized mannans (within the range from11 to 28 aldehyde groups/ mol mannan) and each from these was conjugated at three different ratios of mannan to BSA 1:1, 1,5:1 and 4:1 respectively. In the are shown characteristics of used oxidised mannans (second and third columns) as well as prepared glycoconjugates.
Table 1. Characteristics of Used Mannans and Mannan-BSA Conjugates
As can be seen that Mw of conjugates is dependent on oxidation degree of mannan used for conjugation and also on weight ratio of compounds entering into reaction. The conjugates with highest Mw were obtained from mannan with 20 aldehyde groups per mol mannan prepared at conjugation weight ratio mannan:albumin = 4:1. Suprising was, that the highest content of mannan was determined in conjugate prepared from the least oxidized but the largest mannan (highest Mw).
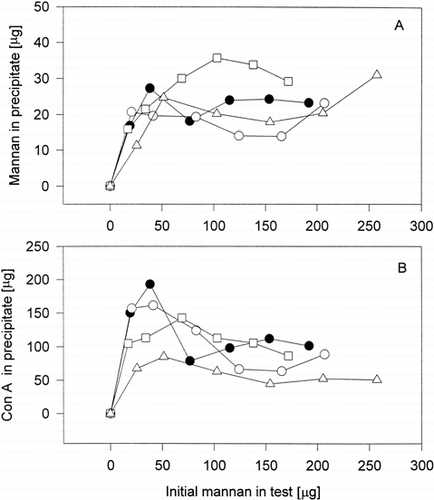
These conjugates, presented in , were tested for their interaction with Con A by using the precipitation method. All samples formed aggregates with Con A, in which the proteins and the saccharides were spectrometrically determined. The comparison of precipitation profiles of original mannan with profiles of three conjugates CM1c – CM3c (prepared at weight ratio mannan: BSA = 4:1) showed quantitaive and qualitative differences (). The most quantitative differences were found between the amounts of Con A determined in aggregates with original mannan and those obtained in aggregates with conjugates (, Panel B). But the amounts of precipitated conjugates expressed as saccharides (, Panel A) are close (in the case CM2c even higher) to that determined at precipitation of original mannan with Con A at the same conditions.
Figure 1. Precipitation profiles of mannan-BSA conjugates (with high content of mannan) with Con A. Panel A – dependence of mannan content (original or in conjugate) in precipitate on initial content of mannan (original or in conjugate) in the test. Panel B – dependence of Con A content in precipitate on initial content of mannan (original) or in conjugate) in the test. -•- original mannan, -○- CM1c, -□ - CM2c, -▵- CM3c.
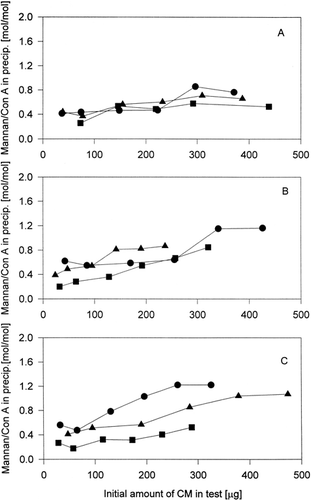
The efficiency of content of mannan in conjugates for their interaction with Con A was better assumed from the dependence of molar ratio mannan/BSA in precipitates on initial amounts of conjugates in precipitation test (). It was found that the small difference in molar ratios mannan (conjugate)/ConA was detected if conjugates with various mannan content were studied. It means that, the higher content of mannan in conjugates with BSA do not have a positive effect on the ratio mannan (conjugate)/Con A in precipitate. It is shown from dependences on Panel A and Panel B of . In the contrast a sizable difference of obtained molar ratio mannan (conjugate) were found at the samples CM3a–c (in which the most oxidised mannan was conjugated) (Panel C), but the dependence on the mannan content in conjugates was not observed. On Panel A and Panel B of is shown the unanbiguous reverse proportion of dependence of molar ratio BSA(conjugate)/Con A in precipitate on initial conjugate amount in precipitation tests on content of mannan in conjugates. In the case of samples CM3a–c (Panel C) was this reverse proportion also observed, but not unanbiguous (the profiles of dependence of ratio conjugate/Con A on initial amount of conjugate in the test of samples CM3b and CM3c were very similar).
Figure 2. Dependence of molar ratio mannan/Con A in precipitate on initial content of mannan-BSA conjugate in the test. Panel A – of conjugates prepared from M1-mannan. Panel B – of conjugates prepared from M2-mannan. Panel C – of conjugates prepared from M3-mannan. -•- CM1a – CM3a, -▪- CM1b–CM3b, -▵- CM1c–CM3c.
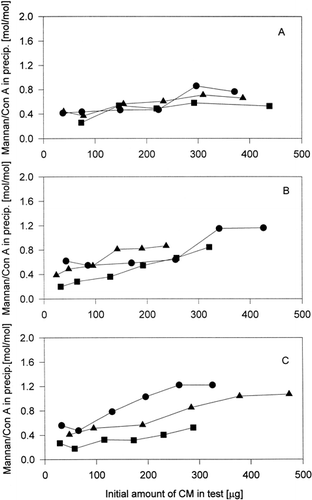
Figure 3. Dependence of molar ratio BSA/Con A in precipitate on initial content of mannan-BSA conjugate in the test. Panel A – of conjugates prepared from M1-mannan. Panel B – of conjugates prepared from M2-mannan. Panel C – of conjugates prepared from M3-mannan. -•- CM1a–CM3a, -▪- CM1b–CM3b, -▵- CM1c–CM3c.
The best results was obtained at the sample CM2a (conjugate prepared from midle oxidised mannan) with content of 28 % of saccharide, where was achieved the molar ratio BSA(conjugate)/Con A(tetramer) as far as value 2.
CONCLUSION
One from many reasons of preparation of neoglycoconjugates from proteins (enzymes) is their affinity to specific lectins. In consequence of saccharide presence in protein (enzymes) molecules these can form aggregates with lectins or bind on the support with immobilized lectin. Resulting from previous it is tendency to prepare the glycoconjugates which are able to bind to lectin, to form the aggregates or to bind on the supports with immobilized lectins, at achievement of higher content of protein (enzyme) in bound form. From our results obtained by using the precipitation method is followed that:
the conjugates prepared from oxidized mannan con taining 20 aldehyde groups per mannan molecule (Mw = 42 kDa) showed the best precipitation behaviours with Con A Citation[[22]]
the interaction of mannan-BSA conjugates with lower content of saccharide (up to 30%) with Con A are more efficacious that those with content of saccharide (above 50%) in point of view of precipitated protein.
ACKNOWLEDGMENTS
This work was supported by the Slovak Grant Agency for Science via grant No.2/5059/98 and 2/1047/21. We would like to thank D.Žišková for the technical assistance.
REFERENCES
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IV.Application of the quantitative precipin method to polysaccharide-Concanavalin A interaction. J. Biol. Chem. 1967; 242: 1617–1622
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. XIII. The interaction of Concanavalin A with α-mannans from a variety of microorganisms. J. Biol. Chem. 1968; 243: 2003–2007
- Mortell K. H., Weatherman R. V., Kiessling L. L. Recognition specificity of neoglycopolymers prepared by ring-opening metathesis polymerization. J. Am. Chem. Soc. 1996; 118: 2297–2298
- Slodki M. E., Ward R. M., Boundy J. A. Concanavalin A as a probe of phoshomannan molecular structure. Biochem. Biophys. Acta 1973; 304: 449–456
- Goldstein I. J., Paretz R. D. Interaction of Concanavalin A with polysaccharides and glycoconjugates. The Lectins, Properties, Functions and Applications in Biology and Medicine, I. E. Liener, N. Sharon, I. J. Goldstein. Academic Press, INC., Orlando, FloridaUSA 1986; 62–67
- Dulaney J. T. Binding interaction of glycoproteins with lectins. Mol. Cell. Biochem. 1979; 21: 43–63
- Mislovičová D., Chudinová M., Gemeiner P., Dočolomansy´ P. Affinity chromatography of invertase on Concanavalin A-bead cellulose matrix: the case of an extraordinary strong binding glycoenzyme. J. Chromatogr. B 1995; 664: 145–153
- Mislovičová D., Stratilová E., Kolarova N. Affinity chromatography of glycoenzymes and glycoproteins on Concanavalin A-bead cellulose. J. Liq. Chrom.& Rel. Technol. 1997; 20: 1367–1379
- Wojczyk B., Hoja D., Litynska A. Structural assessment of β-glucuronidase carbohydrate chains by lectin-affinity chromatography. Glycoconjug. J. 1993; 10: 175–180
- Chui S. H., Lam C. W.K., Lewis W. H.P., Lai K. N. High-performance liquid chromatography for the purification of immunoglobulin A from human serum using jacalin. J. Chromatogr. 1990; 514: 219–225
- Saleemuddin M., Husain Q. Concanavalin A: a useful ligand for glycoenzyme immobilization—a review. Enzyme. Microb. Technol. 1991; 13: 290–295
- Saleemuddin M. Bioaffinity based immobilization of enyzmes. Adv. Biochem. Eng./Biotechnol. 1999; 64: 204–226
- Chadli A., Caron M., Tichá M., Joubert R., Bladier D., Kocourek J. Development of screening methods for detection of carbohydrate-binding proteins by use of soluble glycosylated polyacrylamide-based copolymers. Anal. Biochem. 1992; 204: 198–203
- Bovin N. V., Shiyan S. D., Mikhalchik E. V. New type of carbohydrate–carbohydrate interaction. Glycoconjug. J. 1995; 12: 427
- Pagé D., Zanini D., Roy R. Macromolecular Recognition: effect of Multivalency in the inhibition of binding of yeast mannan to Concanavalin A and pea lectins by mannosylated dendrimers. Bioorg. Med. Chem. 1996; 4: 1949–1961
- Lee R. T., Lee Y. C. Preparation and some biochemical properties of neoglycoproteins produced by reductive amination of thioglycosides containing an ω-aldehydoglycon. Biochemistry 1980; 19: 156–163
- Ek K., Gianazza E., Righetti P. G. Determination of dissociation constants of lectin-sugar complexes and of their pH-dependence by isoelectric focusing electrophoresis. Biochem. Biophys. Acta 1980; 626: 356–365
- Bradley B. K., Ross T. S., Schnaar R. L. Multiple carbohydrate receptors on lymphocytes revealed by adhesion to immobilized polysaccharides. J. Cell. Biol. 1987; 105: 991–997
- Šandula J., Vojtková-Lepšíková A. Immunochemical studies on mannans of the genus Saccharomyces. Group of Saccharomyces sensu sticto species. Folia Microbiol. 1974; 19: 94–101
- Saha S. K., Brewer C. F. Determination of the concentration of oligosaccharides, complex type carbohydrates, and glycoproteins using the phenol/sulfuric acid method. Carbohyd. Res. 1994; 254: 157–167
- Lowry O. H., Rosenbrough N. J., Farr A. L., Smith F. J. Protein measurement with the folin phenol reagent. Biol. Chem. 1951; 193: 265–275
- Masárová J., Mislovičová D., Gemeiner P. Optimization of preparation of dextran and mannan dialdehydes suitable for proteins modification. Chem. Papers, accepted for publication