Abstract
A primary mechanism for Hb mediated vascular contraction appears to be Hb scavenging of endothelium derive nitric oxide (NO), a potent vasodilator. In isolated rat thoracic aorta, however, Hb elicits contraction only after precontraction. The present study investigated a possible role of the alpha adrenergic activation in the Hb mediated contraction. Thoracic aortic rings harvested from normal male SD rats were prepared in a tissue bath and isometric tension changes were evaluated. In vessel rings precontracted with 50nM norepinephrine (NE), 1 μM Hb produced an additional 21.8±13.2% increase in tension. Pretreatment with 70nM phentolamine, an alpha adrenergic antagonist, prevented the 50nM NE induced contraction. In these vessels, subsequent treatment with 2–4μM Hb did not elicit contraction. In vessel rings precontracted with 37mM KCl, 2μM Hb produced an additional 21.8±20.1% tension increase (P<0.05). Pretreatment with phentolamine did neither prevent KCl induced contraction nor affect subsequent Hb mediated additional contraction. To test whether there is a threshold level of basal tension for Hb to trigger contraction, a group of vessel rings were passively stretched to match the tension generated by NE before Hb treatment. In these passively stretched vessel rings, Hb did not produce a significant contraction. Pretreatment with 10mM EGTA, a Ca++ chelator, significantly reduced NE induced contraction (9.7±5.9 vs 137.7±60.0%, P<0.01) but did not prevent it. EGTA also significantly reduced 2μM Hb induced contraction (27.2±29.3% vs 8.9±7.7%, P<0.05). In contrast, pretreatment with verapamil, a Ca++ channel blocker, did not completely block NE and Hb induced contractions. In conclusion, alpha adrenergic activation is not a requisite for the Hb mediated contraction in isolated rat aortic rings. The mechanism how prior tone enhancement allows Hb mediated contraction remains unclear but results from this study suggest a factor that controls cytosolic Ca++ levels may be involved.
INTRODUCTION
Acellular free hemoglobin (Hb), when perfused into the isolated organs or intravenously administered to humans and animals, elicits pressor effects Citation[1-5]. One plausible mechanism for the observed pressor effects is Hb inactivation of endothelium derived nitric oxide (NO), a potent vasodilator. In many species, a low level of NO is believed to be constitutively produced by the vascular endothelium to maintain normal vascular tone Citation[6-7]. Diffused into smooth muscle cells, NO activates cytosolic guanylate cyclase (GC) to produce cGMP which mediates vascular smooth muscle relaxation. Because Hb has a high intrinsic affinity for NO Citation[[8]], infused Hb could react with endothelium derived NO inhibiting the GC- cGMP mediated vascular relaxation pathway. However, in the isolated rat thoracic aorta in the basal state, Hb does not elicit contraction. Hb elicits contraction only in vessel preparations that were precontracted. The most commonly used precontraction agent is an alpha adrenergic pressor agent Citation[6-7], Citation[[9]]. If Hb mediated vasoactivity in the rat thoracic aorta is principally due to NO scavenging, why does Hb fail to elicit contraction in the basal state? Is alpha adrenergic activation a requisite for Hb vasoactivity? Is there a threshold level of vascular smooth muscle tension needed for Hb to elicit contraction? What is the relationship between alpha adrenergic activation and Hb vasoactivity? The present study was initiated to explore these questions.
MATERIALS AND METHODS
Preparation of Rat Aortic Rings and Tension Recording
Male Sprague Dawley rats (250–350g body weight) were anesthetized with sodium pentobarbital (50–75mg/Kg, IP). Through a midline incision, the heart and lungs were removed en bloc. After a wash in Krebs buffer (in mM, NaCl, 118,; KCl, 4.8; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 24; glucose, 11; and disodium EDTA, 0.03; pH=7.4), the thoracic aorta was carefully excised avoiding endothelial damage and placed in a petri-dish containing fresh buffer solution. Following removal of non-vascular tissues, the vessels were cut transversely with a sharp surgical scissors into approximately 2 mm ring segments and kept in Krebs buffer until use. The vessel rings were mounted between two opposing stainless hooks; one hook secured to a tissue holder while the other connected to a tension transducer (Grass Model FT03) via a silk suture (3–0). The vessel ring preparation was placed in a 25ml experimental tissue bath containing a Krebs buffer maintained at 37°C. The buffer was oxygenated continuously by bubbling 95% O2–5% CO2 gas. The vessels were initially set at imposed tension of 2 grams and allowed to relax for 1 hour before experimental treatments were initiated. Change in tension was recorded on a polygraph (Grass Instruments, Quincy, MA).
The responsiveness of vessel rings was first assessed by treating with 50nM norepinephrine (NE); vessel rings that developed less than 0.5g of contraction were considered nonviable and excluded. The integrity of the endothelium was, then, assessed by treating vessels with 10–50 M acetylcholine (Ach). If vessel rings relaxed to Ach less than 10μ% of pretreatment values, functional endothelium was considered absent and were excluded.
Test Hemoglobin Solution
Highly purified human stroma-free Hb (mainly Hb fraction A0) reconstituted in a Ringer's lactate solution (6.4±0.2g Hb/dl) (Hemosol, Inc., Etobicoke, Canada) was used. The Hb solution was aliquoted in 1ml vials and stored frozen -80°C until use. On the day of experiment Hb solution was thawed and diluted as needed with the Krebs buffer before use. Total Hb content and percent of oxyHb and metHb were evaluated with a IL282 Co-Oximeter (Instrument Laboratories, Lexington, MA). Only Hb solution with less than 10% metHb content was used
Drugs and Chemicals
Commercial pharmaceutical preparations of norepinephrine (NE; levarterenol bitartarate, Winthrop Laboratories, New York, NY), phentolamine (Ciba-Geigy Ltd., Basle, Switzerland) and verapamil (Abott Laboratories, North Chicago, IL) were used. Potassium chloride (KCl), acetylcholine chloride (Ach), ethylene glycol aminoethyl ether tetraacetic acid (EGTA) and other chemicals were purchased from Sigma Chemical Co. (Saint Louis, MO).
Statistical Analysis
Data are presented as mean±1 standard deviation (SD). Statistical significance was determined by Student's paired (before and after treatment comparisons) or unpaired t-tests (between group comparisons) at p = 0.05 level.
EXPERIMENTAL PROTOCOLS
Alpha Adrenergic Tone Enhancement and Hb Vasoactivity
To define the role of the alpha adrenergic activation in the Hb mediated vasoactivity, four groups of 6 vessel rings each were pretreated with 0, 0.7, 7.0 or 70nM phentolamine, an alpha adrenergic antagonist, before NE and Hb treatments. Effects of phentolamine doses on NE and Hb induced contraction were assessed and compared with those of phentolamine untreated vessel rings.
Non-adrenergic Pharmacologic Tone Enhancement
Isolated vessel contractions have also been observed following treatment with non-adrenergic contractile agents including KCl, prostaglandin F2α, and se-rotonine Citation[10-11]. Because KCl is a non-receptor mediated contractile agent, Hb contraction of vessel rings precontracted with KCl was investigated. Four groups of vessel rings (N=6–7 each) were precontracted with either 50nM NE or 37mM KCl in the presence and absence of 70nM phentolamine and responses to 2μM Hb were assessed and compared. This dose of phentolamine was chosen because it was shown to completely inhibit the 50nM NE induced contraction.
Passive Mechanical Stretch on Hb Vasoactivity
Is there a physical threshold level of vascular tone below which Hb mediated contraction simply does not occur? To answer this question, two groups of vessel rings (N=5–6 each) were subjected to mechanical stretch or pharmacologic contraction and contractile responses to Hb were assessed. Each experimental run was conducted with a pair of vascular rings prepared from adjacent regions of the same vessel; one was tone enhanced with 50 nM norepinephrine and the other mechanibcally stretched. The tension developed by the mechanically stretched vessel ring was manually adjusted to match that of norepinephrine treated vessel ring. Once vessel rings were stabilized at the desired tension level, 1μM Hb was added to both vessel rings and contractile responses compared.
Role of Calcium Ion
Vascular smooth muscle contraction requires an increase in cytosolic free Ca++ Citation[[12]]. Both alpha adrenergic agonist and KCl induced contractions are known to involve mobilization of Ca++. To assess the role of Ca++ ions in the Hb mediated contraction, two group of vessel rings (N=6 each) were tested in the absence or presence of 10 mM EGTA (a dose required to chelate all free Ca++ in the buffer), a calcium chelator. In addition, to determine whether Ca++ is transported from extracellular sources or arisen from the internal storage, two separate groups of 6 vessel rings were pretreated with or without 20 μM verapamil, a Ca++ channel blocker, and subsequent Hb vasoactivity assessed.
RESULTS
Alpha Adrenergic Tone Enhancement and Hb Vasoactivity
While vessel rings treated with Hb in the basal state do not contract, Hb treatment elicits a further contraction in vessel rings precontracted with norepinephrine(NE) (a). Pretreatment with phentolamine, an alpha adrenergic antagonist, inhibited NE induced contraction (b). In these vessel rings, subsequent Hb as high as 4 μM did not elicit contractions. The phentolamine inhibition of NE and Hb mediated contractions were dose dependent (). Phentolamine doses at 7 nM significantly attenuated the 50 nM NE induced contraction (P<0.05). Subsequent Hb mediated additional contraction was also significantly attenuated compared with the values without phentolamine pretreatment. Pretreatment with 70 nM phentolamine virtually abolished the NE induced contraction as well as subsequent Hb mediated contraction.
Figure 1. A typical responses of endothelium intact rat aortic rings to norepinephrine (NE) and subsequent hemoglobin (Hb). (a) In vessel rings precontracted with 50nM NE, 4μM Hb elicits an additional contraction. (b) In vessel rings pretreated with 70nM phentolamine, an alpha adrenergic antagonist, no notable contraction resulted with 50nM NE. Subsequent Hb treatment does not elicit contraction.
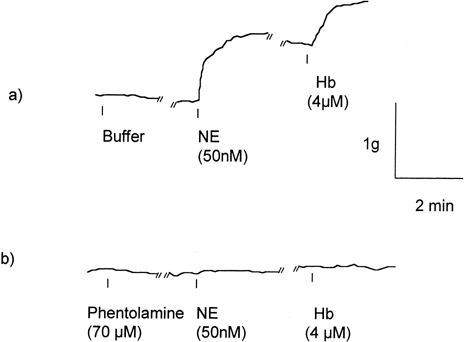
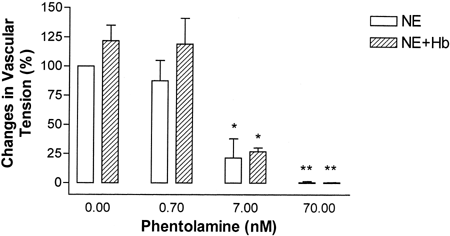
Figure 2. Effect of phentolamine doses on 50 nM norepinephrine (NE) and 2μM Hb mediated vessel ring contractions. Values are presented as percentage of control vessel ring tension produced by 50 nM NE (mean±1 SD, N=6 each). * P<0.05, ** P<0.01 compared with respective control vascular tension without phentolamine pretreatment.
Non-adrenergic Tone Enhancement on Hb Mediated Contraction
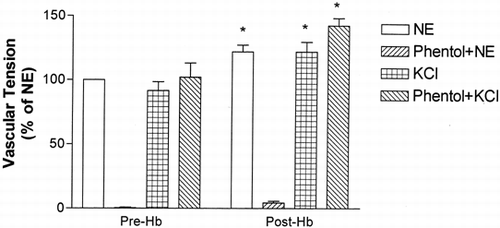
Treatment with 37mM KCl produced vessel ring contraction comparable to that generated by 10nM norepinephrine; subsequent treatment with Hb elicited a further contraction (). This KCl induced contraction was not affected by phentolamine pretreatment. In vessel rings precontracted with 37mM KCl, 2 μM Hb produced an additional 21.8±20.1% (P<0.05) tension increase. While 70 nM phentolamine almost completely inhibited the NE induced contraction, it did not prevent the 37mM KCl induced contraction; subsequent Hb treatment caused an additional tension increase of 42.3±15.5% (P<0.05, ).
Figure 3. A typical vessel ring responses to 37 mM KCl and 4μM Hb. Unlike NE induced contraction, pretreatment with 70nM phentolamine does not alter the KCl induced contraction. Subsequent 4μM Hb treatment elicits an additional contraction.
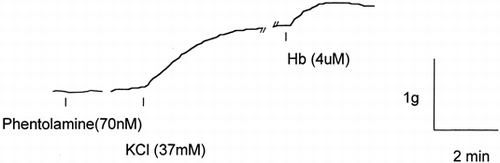
Figure 4. Precontraction with 50 nM norepinephrine (NE) or 37 mM KCl and vessel ring tension responses to 2 μM Hb with or without pretreatment with 70 nM phentolamine (Phentol). Values are represented as percent (mean±1 SD, N=6–7 each) of vessel ring tension responses to NE without phentolamine. * P<0.05 compared with respective pretreatment values.
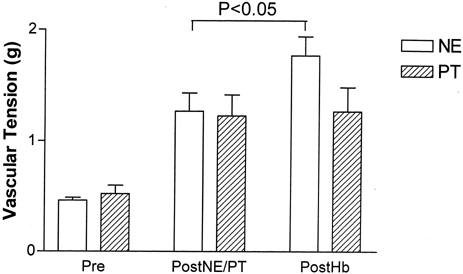
Pharmacologic Versus Mechanical Tone Enhancement
A pair of vessel rings were used for each experimental run; one vessel ring was treated with a standard NE dose (50 nM) and the other mechanically stretched to match the tension of NE treated vessel. When the steady state tensions reached an approximately equal level, both vessel rings were treated with 1 μM Hb. For vessel rings with 0.5g resting tension, 50nM NE increased vascular tension to 1.27±0.36 g (P<0.01). The other groups of vessel rings were passively stretched to match the tension of the NE treated vessel rings. In NE vessel rings, subsequent 1 μM Hb treatment elicited a significantly increased vascular tension to 1.77±0.38 g (P<0.01) while passively stretched vessels showed no significant increase ().
Figure 5. Effect of pharmacologically induced (NE) or passively induced vascular tension (PT) on subsequent vessel ring responses to Hb. While in vessel rings precontracted with 50nM NE, 2μM Hb elicits a significant additional contraction, in vessel rings passively stretched to match the tension, 2 μM Hb did not elicit a significant contraction. Values are in mean±1 SD (N=5–6 each).
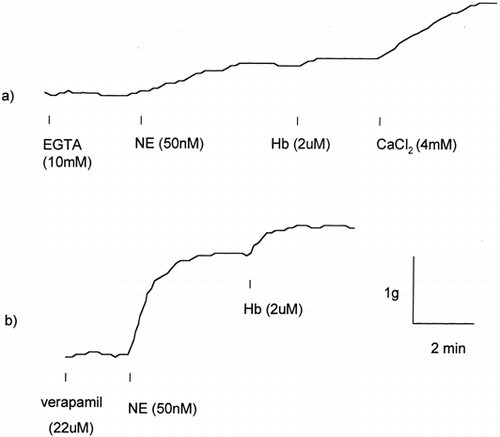
Role of Calcium Ion
Pretreatment with 10mM EGTA attenuated but not completely inhibited the 50nM NE induced contraction (a). The EGTA pretreatment also attenuated vessel ring responses to subsequent Hb. Vessel rings pretreated with 10mM EGTA showed a significant reduction in NE induced contraction compared with buffer treated control vessel rings (9.7±5.9% versus 137.7±60.0%, respectively, P<0.01). Responses to 2μM Hb were also significantly reduced in EGTA treated vessel rings compared with controls (8.9±7.7% versus 27.2±29.3%, P<0.05). In these vessels, the subsequent addition of 4mM CaCl2 restored the Hb mediated contraction resulting in 12.5±10.4% increase in tension over the pre-Hb value. Pretreatment of vessel rings with verapamil, a Ca++ channel blocker, did not completely prevent contraction induced by either norepinephrine or subsequent Hb (Figure-b). Pretreatment with 22 μM verapamil significantly reduced but did not prevent 2μM Hb induced contraction when compared with verapamil untreated controls (29.0±21.4% versus 61.5±21.1%, respectively, N=4 each, P<0.05)
Figure 6. Effect of Ca++ on vessel ring responses to norepinephrine (NE) and Hb. (a) In the presence of 10mM EGTA, vessel ring contractile responses to NE and Hb were notably attenuated. Subsequent addition of 4mM CaCl2 restored the contraction. (b) Calcium channel blocker verapamil as high as 22μM did not alter the NE and Hb induced vessel ring contractile responses.
DISCUSSION
In isolated rat aortic rings, Hb mediated contractions have been observed only when vessels were precontracted (tone enhancement). The most commonly used precontraction agents were alpha adrenergic agonists such as NE or phenylephrine Citation[6-7], Citation[[9]]. This study was initiated to investigate the relationship between the alpha adrenergic mechanism and Hb vasoactivity in the isolated rat thoracic aorta.
The observation that phentolamine, an alpha adrenergic blocker, prevented Hb mediated contraction suggests that an alpha adrenergic pathway may be involved in the Hb mediated contraction in rat thoracic aortic rings. In a recent study, however, a hypothesis that Hb may directly stimulate NE release has been rejected Citation[[13]]. Yet, another study, based on the finding that diaspirin crosslinked Hb augments the adrenergic agonist induced blood pressure elevation cervical sectioned rats, suggests that Hb may sensitize peripheral adrenergic receptors Citation[[14]].
Interestingly, in our study, phentolamine did not prevent the Hb induced contraction in vessel rings precontracted with KCl, suggesting that prior alpha adrenergic activation is not the only condition that allows Hb mediated contraction. Rather, a common mechanism between alpha adrenergic activation and KCl mediated contraction may be involved.
That there may be a threshold basal tension level below which Hb will not elicit contraction (“threshold hypothesis”) was not supported in this study. Passive elevation of vascular tension was not sufficient for Hb induction of contraction in rat thoracic aortic rings. This suggests that an active state of smooth muscle contraction, induced by either a pharmacologic agonist or a neural stimulus, may be necessary for Hb mediated additional contraction.
We, next, investigated whether intracellular or extracelluar Ca++ was utilized in the Hb mediated contraction. In the presence of EGTA, a Ca++ chelator, norepinephrine and KCl did not produce significant contraction in rat aortic rings. Subsequent Hb treatment failed to produce a significant contraction. Only after the addition of an excess of Ca++ was Hb mediated contraction restored suggesting an essential role for Ca++ in vessel ring contraction. While EGTA almost completely inhibited NE and Hb induced contraction, verapamil, a L-type Ca++ channel blocker, only partially inhibited vessel ring responses to NE and Hb. These observations suggest that Hb mediated vascular contraction may occur in part through release of intracellularly stored Ca++ rather than solely from an influx of extracellular Ca++. Little is known about the effect of Hb on intra- and extracellular Ca++ transport and release in vascular smooth muscle cells. Further studies are needed to elucidate possible role of Hb in inter- and intra-cellular Ca++ transport and its relationship to smooth muscle contraction.
A currently postulated basic mechanism of vascular smooth muscle contraction involves an interaction of Ca++ and myosin. A stimulus induced increase in myoplasmic Ca++ activates myosin light chain kinase (MLCK) via a Ca++- calmodulin complex. The resulting myosin regulatory light chain (MRLC) phosphorylation causes an increase in crossbridge cycling and force development Citation[15-16]. Thus, for muscle contraction to occur, myoplasmic Ca++ should increase either by release from internal stores or influx from extracellular sources. It is plausible that Hb alone may not cause a sufficient rise in myoplasmic Ca++ necessary for contraction. Therefore, only after treatment with an agent that increases cytosolic Ca++, could Hb elicit contraction. Pretreatment with contractile agents such as NE, KCl or other similar agents may satisfy this condition. Indeed, both NE and KCl are reported to increase Ca++ influx Citation[[17]].
It is also possible that Hb mediated contraction does not occur in the basal state simply because there may be little or no NO in the unstimulated rat thoracic aorta. In the absence of NO, Hb cannot cause contraction because there is no NO to scavenge. In many species, vascular endothelial nitric oxide synthase is believed to be constitutively active to produce low levels of NO in the basal state. However, there is no published data on basal NO levels in unstimulated rat thoracic aorta. Porcine pulmonary vessels are known to contract with Hb without prior treatment with NE or KCl Citation[[18]]. The higher cGMP levels in these vessels suggest higher basal NO levels to interact with Hb. If this is true, then, why does Ach or organic nitrate pretreatment (presumably stimulating NO production and increasing cGMP level) not allow contraction when vessel in the basal state were treated with Hb? Clearly, further studies are needed to explore these questions.
A recent study with isolated blood vessels revealed that alpha adrenergic activation may also stimulate vascular NO release, in part, through stimulation of beta adrenergic receptors Citation[[19]]. This may explain why Hb elicits an additional contraction following alpha adrenergic agonist induced contraction but still does not explain how Hb mediated additional contraction in vessel rings precontracted with non-adrenergic contractile agonists such as KCl. Clearly, further studies are needed to investigate these issues.
Some Hb based oxygen carriers are reported to elicit pressor effect following intravenous infusion without a prior contractile stimulus Citation[20-23]. Under such conditions, Hb may elicit vascular contraction because there is an active vascular tone maintained by plasma catecholamines and other tonal stimuli Citation[[24]].
In summary, in endothelium intact vessel rings, precontraction with either an alpha adrenergic agent or a vascular depolarization agent allows the Hb mediated contraction. Both alpha adrenergic activation and depolarization induced vascular contraction require an increase in cytosolic free Ca++ level. We, thus, conclude that, in the isolated rat thoracic aorta, the alpha adrenergic agonist induced precontraction may provide a sufficient condition for the Hb mediated contraction to occur but is not a necessary condition. The Hb mediated contraction appears to occur in vessel rings preconditioned to generate an active smooth muscle tone. The alpha adrenergic activation or depolarization may be just some of possibly many such conditions that induce an active vascular tone. The mechanism how the prior tone enhancement allows the Hb mediated contraction remains unclear but may involve regulation of cytosolic Ca++ level.
REFERENCES
- Savitsky P. J., Doczi J., Black J., Arnold J. D. A clinical safety trial of stroma-free hemoglobin. Clinical Pharmacological Therapy 1978; 23: 73–80
- Kim H. W.Rowley B. A., Feola M., Roberts L. A. The effect of stroma-free hemoglobin solution on the isolated perfused rabbit ventricular septum. Fed. Proc. 1983; 42(4)765
- Vogel M. W.Dennis R. C., Cassidy G., Apstein C. S., Valeri C. R. Coronary constrictor effect of stroma-free hemoglobin solutions. Am. J. Physiol. 1986; 251: H413–H420
- Hess J. R.Macdonald V. W., Brinkley W. W. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. Am. J. of Physiol. 1993; 74: 1769–1778
- Collins P., Burman J., Chung H., Fox K. Hemoglobin inhibits endothelium-dependent relaxation to acetylcholine in human coronary arteries in vivo. Circulation 1993; 87: 80–85
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective block-ade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J. Pharmacol. Exp. Therapeutics 1984; 232: 708–716
- Martin W., Smith J. A., White D. G. The mechanisms by which hemoglobin inhibits the relaxation of rabbit aorta induced by nitrovasodilators, nitricoxide, or bovine retractor penis inhibitory factor. J. Pharmacol. 1986; 89: 563–571
- Gibson Q. H., Roughton F. J.W. The kinetics and equilibria of the reactions of nitric oxide with sheep hemoglobin. J. Physiol. 1957; 136: 507–526
- Kim H. W., Greenburg A. G. Pharmacodynamic characterization of hemoglobin induced vasoactivity in isolated rat thoracic aorta. J. Lab. Clin. Med. 2000; 135: 180–187
- Martin W., Furchgott R. F.Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium- derived relaxing factor. J. Pharmacol. Exp. Therap. 1986; 237: 529–538
- Rioux F., Dreapeau G., Marceau F. Recombinant human hemoglobin (rHb1.1) selectively inhibits vasorelaxation elicited by nitric oxide donors in rabbit isolated aortic rings. J. Cardiovasc. Pharmacol. 1995; 25: 587–594
- Katz A. M. Molecular biology of calcium channels in the cardiovascular system. Am. J. Cardiol. 1997; 80: 17I–22I
- Hunter L. W., Tyce G. M.Rorie D. K. Norepinephrine release during va soconstriction induced by cross-linked hemoglobin. Life Sci. 1996; 59: 131–140
- Gulati A., Rebello S. Role of adrenergic mechanisms in the pressor effect of diaspirin cross-linked hemoglobin. J. Lab. Clin. Med. 1994; 124: 125–33
- McDaniel N. L.Rembold C. M., Murphy R. A. Cyclic nucleotide dependent relaxation invascular smooth muscle. Can J. Physiol. Pharmacol. 1993; 72: 1380–1385
- Walsh M. P. Regulation of vascular smooth muscle tone. Can J. Physiol. Pharmacol. 1993; 72: 919–936
- Sward K., Josefsson M., Lydrup M. L., Hellstrand P. Effects of metabolic inhibition on cytoplasmic calcium and contraction in smooth muscle of rat portal vein. Acta Physiol. Scand. 1993; 148: 265–272
- Muldoon S. M.Ledvina M. A.Hart J. L.MacDonald V. W. Hemoglobin- induced contraction of pig pulmonary veins. J. Lab. Clin. Med. 1996; 128: 579–584
- Priest R. M., Hucks D., Ward J. P.T. Noradrenaline, beta-adrenoreceptor mediated vasorelaxation and nitric oxide in large and small pulmonary arteries of the rat. Br. J. Pharmacol. 1997; 122: 1375–1384
- Feola M., Simoni J., Angelillo R., Luhruma Z., Kabakela M., Manzombi M., Kaluila M. Clinical trial of a hemoglobin based blood substitute in patients with sickle cell anemia. Surgery, Gynecology & Obstetrics 1992; 174: 379–386
- Shoemaker S., Gerber M., Evans G., Paik L., Scoggin C. Initial clinical experience with recombinant human hemoglobin. Proceedings of the Vth International Symposium for Blood Substitutes. 1993
- Dunlap E., Farrell L., Nigro C., Estep T., Marchand G., Burhop K. Resuscitation with diaspirin crosslinked hemoglobin in a pig model of hemorrhagic shock. Art. Cells, Blood Subs. and Immob. Biotech. 1995; 23: 39–61
- Johnson J. L.Moore E. E.Offner P. J.Haenel J. B., Hides G. A., Tamura D. Y. Resuscitation of the injured patients with polymerized stroma-free hemoglobin does not produce systemic or pulmonary hypertension. Am. J. Surg. 1998; 176: 612–617
- Pott F., Jensen K., Hansen H., Christensen N. J.Lassen N. A. Middel cerebral artery blood velocity and plasma catecholamines during exercise. Acta Physiol. Scand. 1996; 158: 349–356