Abstract
No intravenously injectable enzyme preparate containing urease as an alternetive to hemodialysis, hemoperfüsion and CAPD systems in patients having chronic renal failure has been encountered in literature. In this study, it has been aimed to convert blood urea to alanine by using PEG-urease/PEG-AlaDH enzyme pair encapsulated within living erythrocyte. In this system, urea is decomposed into NH3 and HCO3- and the ammonia released is converted into alanine by reacting pyruvate under the catalytic action of alaninedehydrogenase. The production of pyruvate and NADH by erythrocyte required in the second stage of the reaction will make the process a feasible and ceaseless one. The success of the system will enable the renal patients with diabetes mellitus. Urease and AlaDH were covalently immobilized on activated PEG. PEG- urease / PEG-AlaDH were encapsulated in erythrocyte (1/1)(v/v) by using slow dialysis methods. The activity of enzyme system, encapsulation yield and hemogram analysis were determined for each sample.
INTRODUCTION
Some molecules can be inserted into erythrocytes by dialyzing or by diluting the suspension of erythrocytes and compound with a hypotonic solution and lowering the osmotic concentration of the medium surrounding the cells. As a result of this, erythrocytes become swollen and numerous holes of sufficient size are formed on the cell membrane. Intracellular components such as enzymes, proteins and drugs which are impermeant solutes at normal condition, can enter into the cells. When isotonicity is restored, the openings on the cell membrane close and some of the molecules is trapped within the erythrocytesCitation[[1]]
Many researches have encapsulated molecules in erythrocytes from different mammals and man by various modifications of the above procedure Citation[2-10]. Encapsulation of molecules in red blood cell have been reported to serve as a potential biodegradable cellular carrier for the in vivo delivery of therapeutic compounds Citation[[11]], Citation[[5]], Citation[12-13]. This would protect these compounds from premature degradation and loss of pharmacological activity Citation[14-15]. Erythrocytes employed as drug carriers are stable and remain in the circulation from 10 to 30 days depending on the animal species Citation[16-19].
Recently applications of immobilized urease for medical use have been extensively studied and devoloped as the basis of urease to replace the bulky and expensive hemodialysis machine Citation[20-23]. Most of these immobilized urease preparation are used for extracorporeal blood circulation Citation[24-27], oral administration Citation[28-29] and peritonal enjection Citation[[26]]. No intravenously enjectable enzyme preparate containing urease in patients having chronic renal failure has been encountered in literature.
The aim of this study, is to prepare easily enjectable, stable and nonimmunogenic urease conjugate by using erythrocytes as a carrier. Urease and AlaDH were immobilized on activated PEG and PEG-urease/PEG-AlaDH enzyme system was encapsulated within living erythrocyte and the ability of alanine synthesis in this systems were investigeted.
MATERIALS AND METHODS
Chemicals
Urease(type III), alaninedehydrogenase(AlaDH), pyruvate, nicotineamidedinucleotide (NADH), methoxypolyethyleneglycol- 5000(MPEG) were obtained from Sigma Chemical; phenol, urea, trichloro-s-triazin and dinitrophenilhydrazin were purchased from Merck Darmstad. All other chemicals and organic solvent were obtained from commonly used suppliess and analytical grade or better.
Preparation of PEG-Urease and PEG-AlaDH
MPEG-5000 was activated with cyanuric chloride and coupled to urease and AlaDH by our previously study Citation[30-31].
Urease Activity
The ammonia formed is estimated according to Berthelot method Citation[32-33]. The color intensity of the complex formed as determined at 630 nm and the activities were calculated by using standart curve (0−10.5 μmol/ml NH4+/ml).
AlaDH Activity
AlaDH and PEG-AlaDH activity were determined as described by Sidney and Kaplan Citation[[34]]. AlaDH was assayed by monitoring initial rates of NADH oxidation at 340 nm in a Jasco spectrophotometer. The standart assay mixture for reductive amination contained 200 μl(17.6 mg/ml) pyruvate, 50 μl(10 mg/ml) NADH, 150 μl(16.05 mg/ml) NH4Cl and 50 mM phosphate buffer (pH 8.0) and enzyme in a final volume of 3.0 ml. One unit of the enzyme defined as the amount of enzyme that catalyzes the formation of 1 mmol NADH per min.
Preparation of Red Blood Cell
Human erythrocytes were isolated from freshly drawn, heparanized verous blood. Whole blood was centrifuged at 1000xg for 10 min. The serum and buffy- coat were removed and the packed cells were washed three times in 10 vol of phosphate buffer saline (10 mM pH 7.4; 0.15 M NaCl, 10 mM glucose and 10 mM inosine)(PBS)Citation[[31]].
Encapsulation of PEG-Urease/ PEG-AlaDH
PEG-urease and PEG-AlaDH were encapsulated within erythrocytes as described by Kruse et al. with optimization by our previously study Citation[[31]] using 0.5U PEG-urease: 1.5U PEG-AlaDH ratio.
Encapsulation Yield
After encapsulation, the suspension washed three times with PBS buffer and centrifuged 2000xg for 5 min and collected centrifugate. PEG-AlaDH and PEG-urease were investigated in washed water and encapsulation yield were determined.
Activity of Enzyme System
After encapsulation, three different tube were prepared for determination of percentage entrapment, enzyme system activity and ability of alanine synthesis in this systems:
Blank; 100 μl natural erythrocyte and 2.9 ml 10 mM citrate phosphate dextrose buffer(CPD) pH 7.6Citation[[31]].
Control; 100 μl erythrocyte including PEG-urease/PEG-AlaDH enzyme system, 10 μl urea (0.417 μmol/ml) and 2.89 ml CPD buffer.
Sample; 100 μl erythrocyte including enzyme system, 200 μl pyruvate (8.34 μmol/ml), 50 μl NADH (0.846 μmol/ml), 10 μl urea (0.417 μmol/ml) and 10 mM pH 7.6 CPD buffer in a final volume 3.0 ml.
All tubes incubated 37°C at different time (5, 10, 15, 20, 30, 45, 60 and 90) and after each incubation time, the samples were centrifuged at 2000xg for 5 min and remove erythrocytes and store centrifugate at 4°C for determination the level of pyruvate and ammonia. Pyruvate was assayed by dinitrophenilhydrozon methods as described by Aras (standart curve 0–30 μg pyruvate/ml). The ammonia level is estimated according to Berthelot method Citation[32-33] by using standart curve 0–10.5 μmol NH4/ml.
Hematologic Parameters
Hematologic determinations including RBC count, mean cell volume (McV), hematocrit (HcT), mean cell hemoglobin (McH) and mean corpuscular hemoglobin (McHc) were done on a hematological analyser Celldyn 4000.
RESULT AND DISCUSSION
Encapsulation Yield
PEG-urease and PEG-AlaDH were encapsulated within erythrocytes using 0.5U PEG-urease: 1.5U PEG-AlaDH ratio. Encapsulation yield and the amount of encapsulated enzyme were given in for PEG-AlaDH and for PEG-urease.
Table I. The Encapsulation Yield of PEG-AlaDH
Table II. The Encapsulation Yield of PEG-Urease
As is shown in and 0.44 U/ml PEG-urease and 0.79 U/ml PEG- AlaDH were encapsulated in erythrocyte.
Ammonia Assay
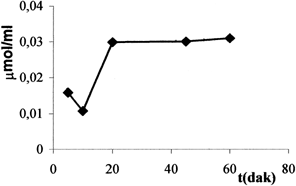
The level of ammonia assemble for each incubation time were given in . 0.03 μmol/ml ammonia were determined at 20 min and this level is very low.
Pyruvate Assay
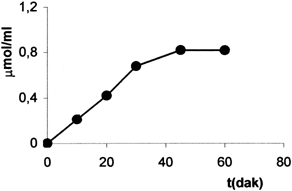
The measurement of pyruvate were given in for each incubation time. The enzyme system activity was estimated using the slope of the linear part of the curve and eq 1:
As is shown in , the consumption of pyruvate is 0.81 μmol/ml at 60 min so the same amount of alanine is synthesis erythrocyte-enzyme system.
Hematologic Parameters
The hematologic parameters of encapsulated and control red blood cell (RBC) were given in . As is shown in , there was a little drop in McV and hemoglobine concentration. Carrier cells are somewhat smaller than normal cells. Since carrier erythrocytes are smaller than normal erythrocytes, they may be less deformable. We obtained cell recovery range %94.12 and a mean percentage of hemoglobin release %15.38.
Table III. Hematologic Parameters
REFERENCES
- Ebrahim A., Ryan W. L. Cytometry 1996; 25: 156–163
- Dale G. L., et al. Biochem. Med. 1977; 18: 220–225
- DeLoach J. R., Ihler G. M. Biochem.Biophys.Acta 1977; 496: 136–145
- DeLoach J. R., et al. Biochem.Biophys.Acta 1977; 496: 507–515
- Ihler G. M. Proc. Nat. Acad. Sci. USA 1973; 70: 2663–2666
- Ihler G., et al. J. Clin. Invest 1975; 56: 595–602
- Thorpe S. R., et al. Pediatr. Res. 1975; 9: 918–923
- updike S. J., et al. Science 1976; 193: 681–683
- Zimmerman U., et al. J. Clin. Chem. Clin. Biochem. 1978; 16: 135–144
- DeLoach J. R., et al. Anal. Biochem. 1980; 102: 220–227
- Fiddler M. B., et al. J. Lab. Clin. Med. 1980; 96: 307–317
- Pitt E., et al. Biochem. Pharmacol. 1983; 32: 3359–3368
- Leung P., et al. Toxicol Appl. Pharmacol. 1986; 83: 101–107
- Naqi A., et al. Biotechnol. Appl. Biochem. 1988; 10: 365–372
- Kojima K., et al. Am. J. Clin. Pathol. 1989; 92: 57–61
- DeLoach J. R. Am. J. Vet. Res. 1983; 44: 1159–1161
- DeLoach J. R., et al. Am. J. Vet. Res. 1981; 42: 667–669
- DeLoach J. R., et al. J. Appl. Biochem. 1980; 2: 177–182
- Hubbard A. R., et al. Biochem. Soc. Trans. 1980; 8: 578
- Chang T. M.S., et al. Can. J. Phsiol. Pharmacol. 1966; 44: 115–128
- Chang T. M.S. Kidney Int. 1974; 387–392
- Chang T. M.S. Sep. Purif. Methods 1974; 3: 245–262
- Chang T. M.S. Clin. Nephrol. 1979; 11: 111–119
- Chang T. M.S. Methods in Enzym. 1988 137: 444–457
- Immobilized Enzymes and Cells. Methods in Enzym, K. Mosbach, 1988; 137
- Drug. Enzyme Targeting. Methods in Enzym, K. J. Widder, R. Green, 1988
- Chang T. M.S. Trans. Am. Soc. Artif. Inter. Organs 1966; 12: 13–19
- Bourget L., Chang T. M.S. FEBS Letter 1985; 180: 5–8
- Chang T. M.S., Lister C. Biomater. Artif. Cells Artif. Organs 1988; 16(5)915–926
- Hamarat Ş., Uslan A. H. Artif. Cells Blood Subs. Imm. Biotech. 1996; 24(3)273–283
- Hamarat Baysal Ş., Uslan A. H. Artif. Cells Blood Subs. Imm. Biotech. 2000; 28(3)263–261
- Charey A. L., Marbach E. P. Clin. Chem. 1962; 8: 30
- Klinik Tanida Laboratuvar, H. İmren, 1985; 637
- Methods in Enzym, P. C. S.dney, N. O. Kaplan, 1962; 5: 673