Abstract
[Poly(ethyleneglycol)]-modified hemoglobin vesicles (PEG-HbV), [nba type of encapsulated hemoglobin, have been developed as artificial oxygen carriers and it is important to evaluate their blood compatibility. We studied the effects of PEG-HbV on human polymorphonuclear neutrophils (PMNs) in vitro, focusing on the functional responses to N-formyl-methionyl-leucyl-phenylalanine (fMLP) as an agonist. The pretreatment of the PMNs with PEG-HbV up to a concentration of 60 mg/dl Hb did not affect the fMLP-triggered chemotactic activity. In parallel to these results, the fMLP-induced upregulation of CD11b (Mac-1) levels on the PEG-HbV-pretreated PMNs was comparable to that of untreated cells. Furthermore, the pretreatment of the PMNs with the PEG-HbV even at 600 mg/dl Hb did not affect the gelatinase B (Matrix methalloproteinase-9 (MMP-9)) release, suggesting that the fMLP-induced release of secondary and tertiary granules was normal. In addition, the fMLP-triggered superoxide production of the PMNs was unchanged by the pretreatment with the PEG-HbV at 600 mg/dl Hb. Thus, these results suggest that PEG-HbV, at the concentrations studied, have no aberrant effects on the fMLP-triggered functions of human PMNs.
INTRODUCTION
Hemoglobin vesicles (HbV) or liposome-encapsulated hemoglobin are candidates for red blood cell substitutes that have the cellular structure of phospholipid vesicles containing concentrated Hb Citation[1-4]. These materials have several advantages including an increase in the oxygen-transporting capability by encapsulating an allosteric effector, a longer half-life in the circulation, an adjustment of viscosity and colloidal osmotic pressure, and a restoration of the methemoglobin reduction system of natural erythrocytes by the inclusion of a coenzyme such as methemoglobin reductase Citation[4-10]. The efficacy of HbV as artificial oxygen carriers has been demonstrated in a 40% exchange transfusion with the HbV suspended in saline Citation[[11]] and a 90% exhange transfusion with HbV in the presence of albumin as a plasma expander using rats Citation[[12]]. Moreover, surface modification of HbV with poly(ethyleneglycol)-phosphatidylethanolamine (PEG-DSPE) reduced the viscosity by suppression of inter-vesicular aggregation and offered promt blood circulation in vivo Citation[[13]].
The blood compatibility of the PEG-modified HbV (PEG-HbV) is an important issue for the clinical use of this material. Polymorphonuclear neutrophils (PMNs) are essential cells in the host defence against a variety of infectious agents. Circulating PMNs require activation to migrate to inflammatory sites and then efficiently kill pathogens. Previously, in the field of drug delivery systems, sterically stabilized liposomes with PEG have been reported to reduce the chemotactic activity of human PMNs in response to zymosan and the bacterially-derived peptide, N-formyl-methionyl-leucyl-phenylalanine (fMLP)Citation[[14]]. However, whether PEG-HbV affect the multiple functional responses in human PMNs is not known. Therefore, we aimed to examine the effects of PEG-HbV on the fMLP-triggered responses in human PMNs including chemotaxis, the release of granule contents, and superoxide production.
MATERIALS AND METHODS
PEG-HbV
PEG-HbV suspended in phosphate buffered saline (PBS) were prepared in the same manner as previously described Citation[[13]]. They encapsulated carbonylhemoglobin with pyridoxal 5′-phosphate (PLP) (Merck Co., Germany) at a molar ratio of [Hb]/[PLP] = 1/1 as an allosteric effector and 5 mM DL-homocysteine (Aldrich Chemical Co., MA). The lipid bilayer was composed of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine, cholesterol, and 1,2-dipalmitoyl-sn-glycero-3-phopatidylglycerol at a molar ratio of 5/5/1 (Nippon Fine Chemical Co., Osa-ka, Japan). The surface-modified HbV with PEG was prepared by the use of 1,2-dipstearoyl-sn-glycero-3-phosphatidylethanolamine-N-[poly(oxyethylene-glycol) 5,000] (NOF Co., Tokyo, Japan). The Hb concentration of PEG-HbV was regulated to 10 g/dl. The PEG-HbV was 230 ± 81 nm in diameter.
Isolation of PMNs
The PMNs were isolated from whole blood (20 ml, heparinized [25 μl/ml]) collected by venipuncture from healthy volunteers without any clinical symptoms of inflammation. Briefly, one fourth volumes of 6 g/dl dextran in PBS were added to the whole blood, mixed well and left to stand for 60 min to allow the RBCs to settle. The upper layer was then applied onto a Ficoll-Hypaque in a 15 ml tube and centrifuged at 1500 rpm for 30 min at room temperature. After hemolyzing the RBC contaminated in the precipitated cells by hypotonic shock with ice-cold H2O, the cells were washed twice with an RPMI-1640 culture medium (GIBCO BRL, Gaithersburg, MD) and then suspended in RPMI-1640 at a final concentration of 106 cells/ml. In the case of the assays for gelatinase B release and superoxide production, the cells were washed twice with Hank's Balanced Salt Solution without Ca2+ and Mg2+ [HBSS(−)] containing 0.03 g/dl human serum albumin (HSA) and then suspended in Hank's Balanced Salt Solution with Ca2+ and Mg2+ [HBSS(+)] containing HSA at a concentration of 107 cells/ml. A typical preparation contained more than 95% viable neutrophils.
Chemotaxis Assay
The PMNs (1.0 × 106/ml in RPMI-1640) were treated with the indicated concentrations of PEG-HbV for 30 min at 37°C in a 5% CO2 humidified atmosphere, and then diluted to 3.0 × 105/ml with RPMI-1640. The PMN chemotaxis was quantified using a modification of the Boyden chamber technique Citation[[15]]. Three hundred and fifty microliters of the cell suspension containing 1.0 × 105 PMNs were loaded into each chemotactic chamber (Chemotaxicell, Kurabo, Osaka, Japan) with an attached polyvinylpyrrolidone-free polycarbonate filter (3-μm pore size). A 24-well plate (3047, Falcon), used as the lower chamber, was loaded with 850 μl of RPMI-1640 containing of 1 μM fMLP as a chemoattractant. The chamber was incubated for 30 min at 37°C in a 5% CO2 humidified atmosphere to allow migration of the cells through the filter. After incubation, the filter was removed, fixed with a May-Grünwald solution (Merck, Darmstadt, Germany) for 10 min, stained with a Giemsa solution (Merck) for 15 min and then dried on a glass slide. The migrated cells were counted under a microscope (× 200) in triplicate.
CD11b (Mac-1) Expression
The detection of the CD11b (Mac-1) expression of the PMNs in responseto fMLP was carried out as previously reported Citation[[16]]. The PMNs treated were in-cubated with PE-conjugated CD11b (Mac-1) or isotype control (DAKO Japan, Kyoto, Japan) for 5 min at 37°C and then stimulated with vehicle or fMLP (1 μM) for 10 min at 37°C. The cells were promptly transferred to 4°C, and analyzed using an EPICS-XL flow cytometer (Beckman Coulter, Tokyo, Japan).
Gelatinase B (MMP-9) Release Assay
Briefly, 1.0 × 10 7/ml PMNs suspended in HBSS(+) with 0.3 g/dl HSA were treated with 6 g/dl PEG-HbV for 30 min at 37°C. After washing the cellsby repeated centrifugation, the cells were resuspended in the same solution, and treated with 1 μM fMLP at 37°C for the indicated periods. After the reaction, the samples were centrifuged (15000 rpm; 15 min; 4°C). The resultant supernatant was collected and kept at −40°C until use. The level of gelatinase B (MMP-9) was determined by the human MMP-9 ELISA system (Amersham Pharmacia Biotech, Buckinghamshire, England) in duplicate, according to the manufacturer's recommendations.
Superoxide Production Assay
The superoxide production was detected by a chemiluminescence method. Briefly, 5.0 × 106/ml PMNs suspended in HBSS(+) with 0.03 g/dl HSA were treated with 6 g/dl PEG-HbV for 30 min at 37°C. After washing the cells by repeated centrifugation, the cells were preincubated at 37°C and incubated with 50 μM cypridina luciferin analog (Tokyo Kasei Co., Tokyo, Japan). After the addition of 1 μM fMLP, the chemiluminescence was measured with a chemiluminescence reader (Biolimat LB 9500C: Berthold Japan, Japan).
Statistical Analysis
Statistical analysis was performed using one-way ANOVA with Scheffe multiple comparisons, two-way ANOVA, and a paired t-test. A value of p < 0.05 was considered significant.
RESULTS
Effect of PEG-HbV on the fMLP-Triggered Chemotaxis of PMNs
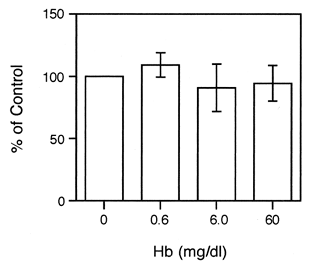
We first studied the effects of PEG-HbV on the chemotactic activity of the PMNs using fMLP as a chemoattractant. The PMNs were treated with various concentrations of PEG-HbV for 30 min, and then allowed to migrate. As shown in , the treatment of the PMNs with the PEG-HbV up to 60 mg/dl of Hb did not cause significant effect on the fMLP-triggered chemotaxis. The treatment of PEG-HbV did not cause a reduction of viability in either the 30 min or 60 min incubation period, as assessed by a trypan blue-exclusion assay (data not shown).
Effect of Pretreatment with PEG-HbV on the fMLP-Induced Release of Granule Contents
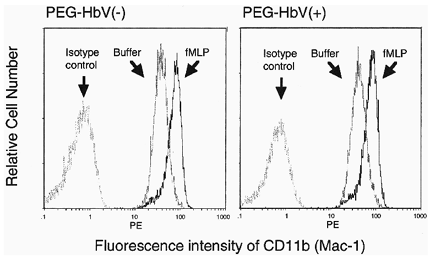
An important functional property of PMNs is their ability to release granule contents in response to agonists. CD11b is the major β2 integrin expressed in the PMNs and is stored in secondary and tertiary granules and secretory vesicles Citation[[17]]. As stimulation of the PMNs with agonists such as fMLP and IL-8 is known to induce upregulation of the cell surface CD11b expression, the effect of the PEG-HbV on the fMLP-induced upregulation of CD11b was examined. The PMNs were preincubated for 30 min with PEG-HbV at 60 mg/dl Hb, and then the expression of CD11b with or without fMLP stimulation was determined by flow cytometry. The stimulation of the control or the PEG-HbV-treated PMNs with fMLP induced a similar upregulation of the cell surface CD11b expression ().
Figure 2. Effects of the PEG-HbV pretreatment on the CD11b (Mac-1) expression on the PMNs. The PMNs were pretreated with PEG-HbV at 60 mg/dl of Hb for 30 min at 37°C. The PMNs were then incubated with 1 μM fMLP or vehicle. The constitutive and fMLP-induced CD11b (Mac-1) expression was analyzed by flow cytometry. Data are representative of two separate experiments.
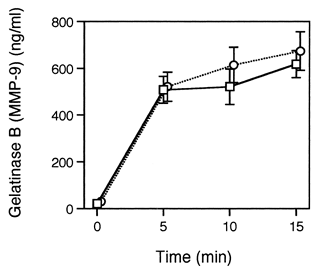
We also measured the gelatinase B (MMP-9) release as another index of the PMN degranulation. Gelatinase B is expressed in the PMNs and is stored in the secondary and tertiary granules Citation[[17]]. The PMNs were preincubated for 30 min with PEG-HbV at 600 mg/dl Hb. After being washed, the PMNs were stimulated with 1 μM fMLP for the indicated times. The levels of gelatinase B released into the supernatant were determined by ELISA. As shown in , the fMLP-induced gelatinase B release in the PEG-HbV-treated PMNs was comparable to the control PMNs. Collectively, these results suggest that the fMLP-induced release of the secondary and tertiary granules is unaffected by pretreatment of the PMNs with the PEG-HbV even at 600 mg/dl.
Figure 3. Effects of the PEG-HbV pretreatment on the gelatinase B (MMP-9) release from the PMNs. The PMNs were preincubated for 30 min with (circle) or without (square) PEG-HbV at 600 mg/dl Hb. After being washed, the PMNs were stimulated with 1 μM fMLP for the indicated times. The levels of gelatinase B released into supernatant were determined by a total MMP-9 ELISA system. Values are means ± SE (N=5).
Effect of Pretreatment with PEG-HbV on the fMLP-Induced Superoxide Production
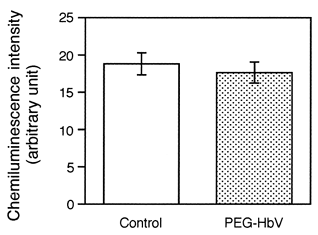
Superoxide production has long been recognized as an important component in host defense. Therefore, we examined the effects of the PEG-HbV on the superoxide production in the PMNs in response to fMLP. To do this, the PMNs were preincubated for 30 min with the PEG-HbV at 600 mg/dl Hb. After being washed, the PMNs were stimulated with 1 μM fMLP. The superoxide production was measured by the cypridina luciferin analog-dependent chemiluminescence. As shown in , there were no differences in the fMLP-triggered superoxide production between the control and the PEG-HbV-pretreated PMNs. Again, this result suggests that the pretreatment of the PMNs with the PEG-HbV even at 600 mg/dl has no significant effects on the fMLP-induced superoxide production.
Figure 4. Effects of the PEG-HbV pretreatment on the superoxide production in the PMNs. The PMNs were preincubated for 30 min with (hatched column) or without (open column) PEG-HbV at 600 mg/dl Hb. After the PMNs were washed, the fMLP (1 μM)-triggered superoxide production was measured by the cypridina luciferin analog-dependent chemiluminescence. Data represent the mean ± SE (N=9).
DISCUSSION
The blood compatible aspect of the PEG-HbV is an important issue for the therapeutic use of this material. We previously studied the effects of the PEG-HbV on the spontaneous and agonist-induced chemokine release from human platelets in vitro, which is regarded as a marker of platelet activation. We found that the presence of high concentration (Hb; 2 g/dl) of PEG-HbV led neither to potentiation nor inhibition of the spontaneous and agonist-induced chemokinerelease Citation[[18]]. To further characterize the effect of PEG-HbV on blood cells, we evaluated whether PEG-HbV have an in vitro effect on WBCs, particularly PMNs. PMNs are known to play essential roles in the host defense against a variety of infectious agents. To kill pathogens efficiently, the circulating PMNs are required to migrate to the inflammatory sites. Recently, in the field of drug delivery systems, Hatipoglu et al. have reported that sterically stabilized liposomes with [poly(ethyleneglycol)2000]-distearoylphosphatidylethanolamine (PEG-DSPE) and PEG-DSPE itself, at 1nM phospholipid concentration, reduced the fMLP and zymosan-induced human PMN chemotaxis in vitro Citation[[14]]. The authors described the inhibitory effects of sterically stabilized liposomes and PEG-DSPE as being specific, because another study has reported that liposomes composed of phosphatidylcholine and phosphatidylserine promoted neutrophil accumulation in the lungs of allergic mice Citation[[19]]. The comparison of these studies suggests that the modification with PEG-DSPE might be a cause of the inhibitory effect of sterically stabilized liposomes on the chemotactic activity of PMNs, although the underlying mechanism of this inhibition has not been identified.
However, we showed that HbV, modified by PEG-DSPE, did not reduce the fMLP-triggered chemotactic activity, despite the fact that much higher levels of phospholipid concentrations (approximately 330 μM) were used. Moreover, the upregulation of the CD11b (Mac-1) expression on the PMNs in response to fMLP was unaffected by the PEG-HbV treatment. The fMLP-induced gelatinase B release was also unchanged by the PEG-HbV pretreament even at 10 times higher concentrations. Although the reason for this disparity is not clear at present, several differences in the physico-chemical properties of the materials used can be pointed out. We used PEG5000 instead of PEG2000, because the larger molecular weight allowed for a smaller molar composition, which is thought to cause less disturbance of the lipid bilayer by the transbilayer asymmetry Citation[[13]]. The difference in the PEG-DSPE to liposome ratio might affect the differential outcome. In addition to the PEG-DSPE involvement, the modification of several parameters such as the composition of the phospholipids, the isomerisms with saturated and unsaturated fatty acids, and the carbon-chain length of the fatty acids have been shown to affect the fMLP and zymosan-induced chemotaxis of PMNs in vitro Citation[[20]]. In fact, these parameters are clearly different between the PEG-HbV and the sterically stabilized liposomes prepared by Hatipoglu et al.
The reduction of oxygen to superoxide anion, a precursor of microbicidal oxidant, is catalyzed by the respiratory burst oxidase, or the nicotinamide adenine diphosphate reduced form (NADPH) oxidase that is a multicomponent enzyme Citation[[21]]. The importance of the oxidase in host defenses is demonstrated by the recurrent and life-threatening infections that occur in patients with chronic granulomatous disease, a hereditary disorder resulting in defective NADPH oxidase activity Citation[22-23]. We found that the superoxide generation in the PEG-HbV-treated PMNs after stimulation with fMLP was comparable to that of the control PMNs, indicating that the NADPH oxidase system in the PMNs is unaffected by pretreatment with the PEG-HbV at the concentrations used. Previously, unsaturated phosphatidylcholines in micromolar concentrations have been shown to inhibit the superoxide production in human neutrophils stimulated by the activators of protein kinase C, phorbol 12-myristate 13-acetate and 1,2-dioctanoyl-sn-glycerol. In contrast, the superoxide production induced by surface receptor agonist fMLP, was unaffected by the phospholipids Citation[[24]]. In this regard, the pretreatment of the PMNs with the PEG-HbV did not inhibit the superoxide production triggered by phorbol 12-myristate 13-acetate either (data not shown).
In conclusion, we evaluated the effects of PEG-HbV on the fMLP-triggered functional responses of human PMNs, and observed that the PEG-HbV, at the concentrations studied, had no significant effects on chemotaxis, the release of granule contents, and superoxide production. These results are of value for estimating the biocompatibility of PEG-HbV for blood cells. Whether the administration of PEG-HbV has an aberrant effect on the functions of PMNs in vivo remains to be determined.
ACKNOWLEDGMENTS
We express our gratitude to the late Dr. Sadayoshi Sekiguchi, the ex-director of the Hokkaido Red Cross Blood Center, for his support of this study. This work was supported in part by a Health Science Research Grant (Artificial Blood Projchect) from the Ministry of Health and Welfare, Japan (#12480268).
REFERENCES
- Djordjevich L., Miller I. F. Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp. Hematol. 1980; 8: 584–592
- Hunt C. A., Burnette R. R., MacGregor R. D., Strubbe A. F., Lau D. T., Taylor N., Kiwada H. Synthesis and evaluation of a prototypal artificial red cell. Science 1985; 230: 1165–1168
- Rudolph A. S. Encapsulated hemoglobin: current issues and future goals. Art. Cells Blood Subs. Immob. Biotech. 1994; 22: 347–360
- Tsuchida E., Takeoka S. Stabilized hemoglobin vesicles. Artificial Red Cells: Materials, Perfomances and Clinical Study as Blood Substitutes, E. Tsuchida. John Wiley and Sons, Chichester 1995; 35–64
- Klibanov A. L., Maruyama K., Torchilin V. P., Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990; 268: 235–237
- Ogata Y., Goto H., Kimura T., Fukui H. Development of Neo Red Cells (NRC) with the enzymatic reduction system of methemoglobin. Art. Cells Blood Subs. Immob. Biotech. 1997; 25: 417–427
- Farmer M. C., Rudolph A. S., Vandergriff K. D., Hayre M. D., Bayne S. A., Johnson S. A. Liposome-encapsulated hemoglobin: oxygen binding properties and respiratory function. Biomater. Artif. Cells Artif. Organs. 1988; 16: 289–299
- Usuba A., Motoki R., Suzuki K., Sakaguchi K., Takahashi A. Study of effect of the newly developed artificial blood ‘Neo Red Cells (NRC)’ on hemodynamics and blood gas transport in canine hemorrhagic shock. Biomater. Artif. Cells Immobil. Biotechnol. 1992; 20: 531–535
- Rabinovici R., Rudolph A. S., Vernick J., Feuerstein G. A new salutary resuscitative fluid: liposome encapsulated hemoglobin/hypertonic saline solution. J. Trauma. 1993; 35: 121–126
- Takaori M., Fukui A. Treatment of massive hemorrhage with liposome encapsulated human hemoglobin (NRC) and hydroxyethyl starch (HES) in beagles. Art. Cells Blood Subs. Immob. Biotech. 1995; 24: 643–653
- Izumi Y., Sakai H., Hamada K., Takeoka S., Yamahata K., Katoh H., Nishide H., Tsuchida E., Kobayashi K. Physiological responses to exchange transfusion with hemoglobin vesicles as an artificial oxygen carrier in anesthetized rats: changes in mean arterial pressure and renal cortical tissue oxygen tension. Crit. Care Med. 1996; 24: 1869–1873
- Izumi Y., Sakai H., Kose T., Hamada K., Takeoka S., Yoshizu A., Horinouchi H., Kato R., Nishide H., Tsuchida E., Kobayashi K. Evaluation of the capabilities of a hemoglobin vesicle as an artificial oxygen carrier in a rat exchange transfusion model. ASAIO J. 1997; 43: 289–297
- Sakai H., Takeoka S., Park S. I., Kose T., Nishide H., Izumi Y., Yoshizu A., Kobayashi K., Tsuchida E. Surface modification of hemoglobin vesicles with poly(ethylene glycol) and effects on aggregation, viscosity, and blood flow during 90% exchange transfusion in anesthetized rats. Bioconjugate Chem. 1997; 8: 23–30
- Hatipoglu U., Gao X. P., Verral S., Séjourné F., Pitrak D., Alkan-Önyüksel H., Rubinstein I. Sterically stabilized phospholipids attenuate human neutrophils chemotaxis in vitro. Life Sci. 1998; 63: 693–699
- Ebina T., Murata K. Antitumor effect of PSK at a distant site: introductions of interleukin-8-like factor and macrophage chemotactic factor in murine tumor. Jpn. J. Cancer Res. 1990; 81: 1307–1313
- Betsuyaku T., Liu F., Senior R. M., Haug J. S., Brown E. J., Jones S. L., Matsushima K., Link D. C. A functional granulocyte colony-stumulating factor receptor is required for normal chemoattractant-induced neutrophil activation. J. Clin. Invest. 1999; 103: 825–832
- Jones D. H., Schmalstieg F. C., Dempsey K., Krater S. S., Nannen D. D., Smith C. W., Anderson D. C. Subcellular distribution and mobilization of MAC-1 (CD11b/CD18) in neonatal neutrophils. Blood 1990; 75: 488–498
- Wakamoto S., Fujihara M., Abe H., Sakai H., Takeoka S., Tsuchida E., Ikeda H., Ikebuchi K. Effects of poly (ethyleneglycol)-modified hemoglobin vesicles on agonist-induced platelet aggregation and RANTES release in vitro. Art. Cells Blood Subs. Immob. Biotech., in press
- Bellemare F., Israel-Assayag E., Cormier Y. PC:PS liposomes induce a recruitment of neutrophils and the release of TNF alpha in the lungs of mice sensitized with Saccharopolyspora rectivirgula. Eur. J. Clin. Invest. 1995; 25: 340–345
- Galdiero F., Carratelli C. R., Bentivoglio C., Capasso C., Cioffi S., Folgore A., Gorga F., Ianniello R., Mattera S., Nuzzo I., Rizzo A., Tufano M. A. Correlation between modification of membrane phospholipids and some biological activity of lymphocytes, neutrophils and macrophages. Immunopharmacol. Immunotoxicol. 1991; 13: 623–642
- Chanock S. J., el Benna J., Smith R. M., Babior B. M. The respiratory burst oxidase. J. Biol. Chem. 1994; 269: 24519–24522
- Roos D. The genetic basis of chronic granulomatous disease. Immunol. Rev. 1994; 138: 121–157
- Thrasher A. J., Keep N. H., Wientjes F., Segal A. W. Chronic granulomatous disease. Biochim. Biophys. Acta. 1994; 1227: 1–24
- Yoshida K., Mohsenin V. Unsaturated phosphatidylcholines inhibit superoxide production in human neutrophils. Life Sci. 1991; 49: 1359–1365