Abstract
A variety of platelet substitutes (e.g. rehydrated lyophilized (RL) platelets thromboerythrocytes plateletsomes infusible platelet membranes synthocytes fibrinogen-coated microcapules) are potentially useful as hemostatic agents in transfusion medicine. However, as “foreign” particles, platelet substitutes interact to varying extents with elements of the reticulo-endothelial system for clearance, reducing hemostatic efficacy. Experiments were performed to better understand the interaction of RL platelets with elements of the innate and acquired immune systems. The infusion of heterologous RL platelets into rats resulted in rapid clearance from the free circulation with half-life values of minutes. The clearance of RL platelets was inhibited when macrophages were rendered apoptotic with gadolinium. Transmission EM analysis of splenic tissue after infusion of lyophilized cells, as well as in vitro mixing studies with splenic macrophages and RL platelets, indicated that macrophage-mediated phagocytosis mechanisms were operant in RL platelet clearance by the reticulo-endothelial system. Studies with IV IgG, as a competitive inhibitor of the macrophage Fc receptor, provides evidence that RL platelet destruction is in part mediated by platelet surface bound IgG. This hypothesis was further supported by the finding that RL platelets react with IgG class antibodies that are pre-existing in na;auive animals. These studies provide a rational basis for prolonging the circulation time of RL platelets and other platelet substitutes.
INTRODUCTION
The experiments reported in this article elucidate mechanistic details of how platelet substitutes interact with elements of the reticulo-endothelial system (RES). Several types of platelet substitutes are being developed (see Blajchman Citation[[1]]) for a recent review)) to address issues related to shortness in supply, the development of storage lesion, microbial contamination and logistical difficulties (see, e.g., Citation[[2]] for a discussion of these issues). We have discovered and refined a method for lyophilizing platelets for long term storage that circumvents many of the problems of stocking fresh platelets Citation[[2]] and yields a cell with native-like membrane adhesion functions Citation[3-5] and intracellular stimulus-response coupling Citation[[6]]. Other substitutes for fresh stored platelets are under development for hemostatic control, including thromboerythrocytes Citation[[7]], plateletsomes Citation[[8]], infusible platelet membranes Citation[[9]], synthocytes Citation[[10]] and fibrinogen-coated microcapsules Citation[[11]].
As a “foreign” particulate, RL platelets and other substitutes for stored fresh platelets interact with elements of the reticulo-endothelial system (RES) for shortened circulation times and reduced efficacy. While many aspects of reticuloendothelial system function are poorly defined, a great deal has been learned from studies involving the maturation and senescence of blood cells, autoimmune disorders of blood cells, immune responses (innate and acquired) to microorganisms and circulation kinetics of artificial substances. The spleen is responsible for “pooling” or “marginalizing” and then phagocytizing foreign particles and senescent blood cells. Splenic pooling of RL platelets might involve epinephrine-reversible interactions with selectins and other adhesion proteins on endothelial cells. Macrophage phagocytosis is mediated by at least four mechanisms that respectively involve the IgG Fc, lectin-like, scavenger, and complement receptors. These mechanisms (see for a summary) potentially function in concert through processes that are primed by chemokine and cytokine responses. The studies we present in this article show that RL platelets are phagocytized by RES macrophages, and that Fc receptor-mediated specific docking mechanisms are involved in the interaction of the lyophilized cells with macrophages for phagocytosis.
Table 1. Mechanisms for Retention and Phagocytosis in the Spleen
MATERIALS AND METHODS
Preparation of Fluorescent Labeled Fresh and RL Platelets from Rats
RL platelets were prepared as detailed elsewhere Citation[[2]]. Briefly, blood was obtained from rats by cardiac puncture, and then platelets were separated from other blood cells and plasma proteins with differential centrifugation. The platelets were cross-linked with paraformaldehyde, frozen and lyophilized. RL platelets were rehydrated and labeled with the green fluorescent thiol label CMFDA at 0.1 uM for 45 min at 37°C. Excess fluorescent label was removed with centrifugation. Fresh rat platelets were labeled with the same procedure after centrifugal washing.
In Vivo Circulation Studies
200 to 250 gram rats (Sprague-Dawley) were anesthetized with ketamine and acepromazine, and then catheterized via the tail vein. Each animal was infused with the equivalent of 10% of its initial total number of endogenous platelets with the CMFDA-labeled (heterologous) RL or fresh platelets. In some experiments, the role of RES macrophages was analyzed by inducing macrophage apoptosis with gadolinium as described by Ruttinger et al. Citation[[12]]. These animals were injected with gadolinium (10 mg/kg) 24 hr before the infusion of RL platelets. In another set of experiments, mechanisms for macrophage phagocytosis were investigated by infusing IV IgG (400 mg/Kg body mass, kindly gifted by Bayer Corp.) or an equivalent amount of normal saline. To measure the level of freely circulating CMFDA-labeled RL or fresh platelets, samples were withdrawn and subjected to flow cytometric analysis to quantify infused cells. RL platelet levels were fit to an exponential function for extraction of a first order rate constant k and related tau or time constant value (1/k). The animal was sacrificed two hours after the infusion, and then the spleen and liver was removed and thin sectioned for fluorescence microscopy as detailed elsewhere Citation[[2]].
In Vitro Phagocytosis
Phagocytosis of CMFDA-labeled RL platelets by splenic macrophages was examined by mixing the platelets and macrophages in tissue culture and then performing fluorescence microscopy and flow cytometry to follow the internalization of RL platelets by the macrophages. To obtain splenic macrophages, the spleen was removed (after euthanasia), flushed with saline remove red blood cells and lymphocytes, and then crushed to free macrophages from the red matter matrix. The resulting mixture of macrophages and other cell types was diluted in DMEM F-12 for 104 macrophages per ml. 1 ml per well of the suspension was placed in 24 well tissue culture plates and then the macrophages were allowed to settle and attach for 6 hr. CMFDA-labeled fresh and RL platelets are added to 1 ml portions of macrophages in the 24 well plates and incubated for 12 hr. at 37°C in a 5% CO2 atmosphere with gentle rocking. Macrophages were cooled to 4°C and labeled with anti-macrophage monoclonal antibody MCA343 (Serotec Ltd, Raleigh, NC) for 1 hr. The supernatant was removed from each well, and then anti-mouse IgG phycoerythrin (red fluorescence) secondary antibody was added and allowed to incubate for 1 hr. Unbound secondary antibody was removed by aspirating the medium twice, and then cells were examined with fluorescence microscopy as detailed elsewhere Citation[[2]]. Portions of the cells were suspended in medium with scraping and EDTA treatment, and then subjected to flow cytometry to quantify the extent of platelet phagocytosis. Macrophages were distinguished from platelets respectively with red and green fluorescence; macrophages that have digested platelets were fluorescent with both green and red probes. Negative and positive controls were performed respectively with autologous fresh rat platelets and fluorescent-labeled human RL platelets.
Detection of Anti-RL Platelet IgG Class Antibodies
108 canine fresh or RL platelets were incubated with 1 ml of canine serum from animals that had not been infused with RL platelets (naïve serum) for 3 hr at room temperature. The RL platelets were then chromatographed on Sepharose 4B to remove unbound IgG and other serum proteins. The platelets were then incubated with anti-canine IgG-FITC and subjected to flow cytometry to measure the level of IgG on the surface of the platelets. Fluorescence intensity was related to surface molecules IgG per platelet by subjecting S. aureus with defined amounts of bound IgG to flow analysis.
RESULTS
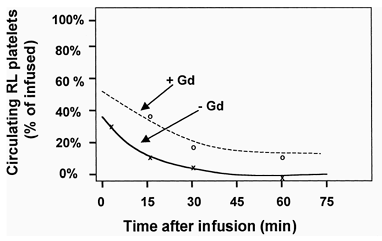
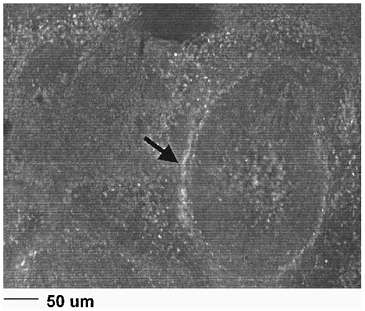
Studies were performed with labeled RL platelets in rats to examine the circulatory clearance kinetics of the lyophilized cells. Rats were infused with heterologous CMFDA-labeled RL platelets equivalent to 10% of the initial endogenous platelet mass. Blood was withdrawn at time points post infusion and subjected to flow cytometric analysis to determine the ratio of fluorescent RL to endogenous platelets. The data in (lower curve) shows that the lyophilized cells were cleared from the circulation with an approximately exponential time course (after an initial rapid drop) with a tau (1/k) value of 9.5 min. When the same experimental protocol was performed after administering gadolinium to render the macrophages apoptotic (e.g., see Mizgerd et al. Citation[[13]]), the circulation of RL platelets was substantially prolonged (, upper curve) for a tau (time constant, 1/k) value of 32.3 min. CMFDA-labeled control platelets yielded tau (1/k) values of over 24 hr (data not shown). The examination of splenic sections two hours after RL platelet infusion with fluorescence microscopy () showed that the RL platelets were closely associated with the endothelial cell/macrophage matrix of splenic cavities.
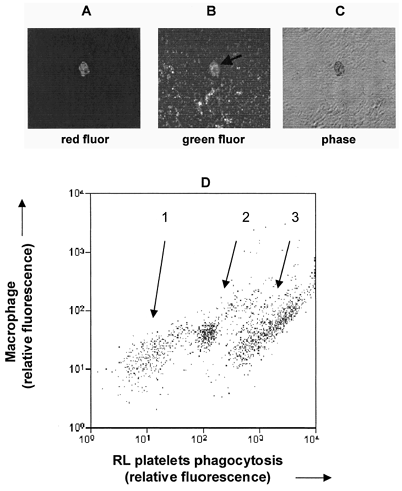
The fate of RL platelets was further examined by performing in vitro phagocytosis studies with splenic macrophages. Splenic macrophages were isolated and incubated with CMFDA-labeled rat RL platelets (green fluorescence). Next the mixtures were labeled with anti-macrophage Mab (for red fluorescence) and analyzed with fluorescence microscopy and flow cytometry. Panels A and B of depict a macrophage that has internalized platelet(s). Free platelets can be seen elsewhere in the field. Flow cytometric analysis of the phagocytosis mixture (Panel D of ) revealed three subpopulations of macrophages with minimal (group 1; 23% of macs), intermediate (group 2, 28% of macs) and high (group 3, 49% of macs) levels of RL platelet uptake. These results, when considered with the sensitivity of clearance to macrophage apoptosis and the deposition of RL platelets splenic cavities, indicate that macrophages of the RES play an important, if not dominant, role in RL platelet clearance.
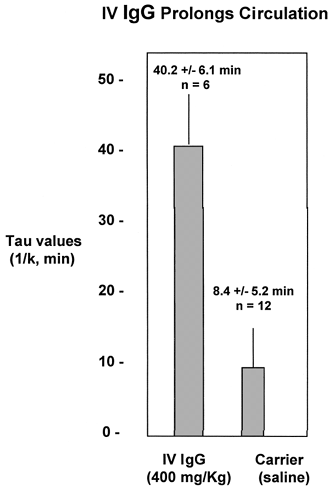
The role of Fc receptor-mediated phagocytosis mechanism in the clearance of RL platelets was examined by performing circulation experiments after pre-infusion of IV IgG to ligate the macrophage Fc receptors before exposure to RL platelets. Rats were infused with 400 mg/kg IV IgG (a standard dose in humans for the treatment of immune thrombyctopenia purpura) five minutes before the infusion of CMFDA-labeled RL platelets. The results in show that IV IgG substantially prolonged the circulation time of the RL platelets from a Tau value of 8.4 min to 40.2 min. This result indicates that macrophage Fc receptor-mediation mechanisms are operant in RL platelet clearance.
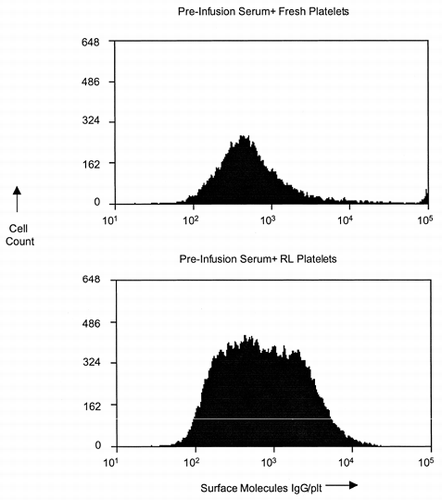
The ability of IV IgG to prolong RL platelet circulation suggests that the lyophilized cells expose an antigenic moiety (a neo/cryptic antigen) as a result of the cross-linking and/or lyophilization process that reacts with pre-existing IgGs in the circulation of naïve animals. Results from our pre-clinical evaluation program in dogs sheds light on this issue. Serums from na;auive dogs (before infusion of any lyophilized platelet products) was incubated with RL and fresh canine platelets, and then surface-bound IgG was measured with flow cytometry. The results presented in show that the surface membrane of fresh platelets bound approximately 500 molecules IgG per cell. This contrasts with RL platelets, which surface exposed increased amounts (up to approximately 104 IgG molecules per cell) of IgG. This result potentially explains why RL platelets are recognized by macrophage Fc receptors for phagocytosis.
Figure 5. Anti-RL platelet IgG is pre-existing in the circulation. 1. RL platelets bind approximately three times more IgG than fresh platelets when incubated with pre-infusion serum. 2. The amount of IgG that binds to each RL platelet is heterodispersed. Flow Cytometric Histograms from Dog X14 Serum incubated with RL or Fresh Platelets.
DISCUSSION
We have shown that RL platelets are cleared from the free circulation in a period of minutes through processes that at (least in part) involve macrophage phagocytosis. Furthermore, the presented data indicates that RL platelets are recognized by IgG class antibodies, and then the IgG opsonized platelets dock to macrophage Fc receptors for phagocytosis. IV IgG inhibits this process through two potential mechanisms. First, IV IgG might function as a competitive inhibitor by binding and occupying the Fc receptor before interaction with RL platelet surface bound IgG Citation[14-15a], Citation[[15b]], see, Daeron Citation[[16]] for a general review). This competitive inhibition is thought to form part of the basis for treating immune-related thrombocytopenias (ITP) with intravenous IgG (IV IgG) Citation[[17]]. Secondly, a component of the IV IgG fraction might act as an anti-idiotypic antibody by binding to anti-RL platelet IgG (e.g., Citation[[18]].
IgG and IgM opsonized cells also interact with serum components of the alternative complement pathway, and then bind to complement receptors on splenic macrophages Citation[19-21]. Complement-mediated binding to macrophages has been inhibited by occluding optimizing complement components with soluble complement receptor mutants (soluble CR1) and anti-complement C5a antibody Citation[[22]]. Fc and complement-receptor bound particles are phagocytized by different mechanisms Citation[[21]]. Fc-mediated phagocytosis results in transport to peroxisomes, activation of respiratory burst and the generation of pro-inflammatory prostaglandins and cytokines Citation[[21]]. In contrast, complement-mediated phagocytosis results in transport to lysosomes for proteo-glycolytic digestion Citation[[21]] with a minimal pro-inflammatory response Citation[23-24]. Fc and complement-mediated phagocytosis might be distinguished by determining if the infusion leads to a pro-inflammatory response and if circulation and phagocytosis is inhibited by soluble complement receptors. Several innate and acquired immune system mechanisms might be involved in the interaction of the RES with RL platelets and other artificial platelet substitutes. In addition to facilitating Fc-mediated macrophage uptake, bound IgG can also activate complement systems Citation[[25]]. The multi-factorial nature of clearance mechanisms is evidenced by an incomplete ability to inhibit clearance with IV IgG, and indicates that a combination of strategies might be required to restore native circulation kinetics.
Our studies demonstrate that a rational pharmacological approach can be used to prolong circulation kinetics of platelet substitutes, and suggests a family of strategies based on inhibiting Fc receptor function. For example, anti-D therapy, which has been used to treat ITP Citation[[26]], might prolong circulation as a competitive inhibitor of IV IgG in Rh-positive individuals. A similar approach could potentially be based on isolation or generation of specific anti-idiotypic antibodies to the antibodies that opsonize platelet substitutes. Alternatively, infusion of recombinant Fc-gamma-receptor fragments, which have been shown to be useful in the treatment of pediatric ITP Citation[[27]], might prolong circulation times by competing with the Fc receptor for the opsonized IgGs. While RL platelets have been shown to be hemostatically effective for hours after clearance from the free circulation Citation[28-29], we anticipate that persistence in the free circulation will result in enhanced ability to control hemorrhage.
ACKNOWLEDGMENTS
This research was supported by Navy Medical Research and Development Command Grants N00014-97-0867 and N00014-97-0891. The authors greatly appreciate the gift of IV IgG from the Bayer Corporation.
REFERENCES
- Blajchman M. Platelet substitutes. Vox Sang. 2000; 78: 183–186
- Read M., Reddick R., Bode A., Bellinger D., Nichols T., Taylor K., Smith S., McMahon D., Griggs T., Brinkhous K. Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: potential for long-term storage of dried platelets for transfusion. Proc. Natl. Acad. Sci. USA 1995; 92: 397–401
- Sanders W., Reddick R., Nichols T., Brinkhous K, Read M. Thrombotic thrombocytopenia induced in dogs and pigs. The role of plasma and platelet vWF in animal models of thrombotic thrombocytopenic purpura. Arterscl., Thromb., and Vasc. Biol. 1994; 15: 793–800
- Khandelwal G., Sanders W., Bode A., Nichols T., Erickson G., Read M. von Willebrand factor binding to rehydrated lyophilized platelet surface GP1b and inhibition by monoclonal antibody to GP1b. FASEB J. 1997; 11: 1812
- Merricks E., Nichols T., Russell K., Read M. Binding and internalization of fibrinogen and F-actin distribution in rehydrated lyophilized platelets. Blood 1998; 10(S1–2)71b
- Fischer T., Merricks E., Russell K., Raymer R., White G., Bode A., Nichols T., Read M. Intracellular Signalling in Rehydrated, Lyophilized Platelets. Brit. J. Haem. 2000; 111: 167–175
- Coller B., Springer K., Muthusamy M., Scudder L., Narla M., Beer J. Further invitro characterization of thromboerythrocytes (TE), a potential autologous, semiartificial platelet substitute. Throm., and Haem. 1991; 65: 755
- Rybak M., Renzulli L. A liposome based platelet substitue, the plateletsome, with hemostatic efficacy. Biomat. Art. Cells Imm. Biotech. 1993; 21: 101–118
- Chao F., Kim B., Houranieh A., Liang F., Konrad M., Swicher S., Tullis J. Infusible platelet membrane microvesicles; a potential transfusion substitute for platelets. Transfusion 1996; 36: 536–542
- Middleton S., Harris R., Davies A., de Groot P., Armstrong D., Gadson D., Levi M. Preliminary characterization of a novel platelet substitute, synthocytes™ for the treatment of thrombocytopenia. Brit. J. Haem. 1998; 101: 266
- Levi M., Friederich P., Middleton S., de Groot P., Wu Y., Harris R., Biemond B., Heijnen H., Levin J., Cate J. Fibrinogen-coated albumin microcapsules reduce bleeding in severely thrombocytopenic rabbits. Nature Med. 1999; 5: 107–111
- Ruttinger D., Vollmar B., Wanner G., Messmer K. In vivo assement of hepatic alterations following gadolinium chloride-induced Kupffer cell blockade. J. Hepatology 1996; 25: 960–967
- Mizgerd J., Molina R., Stearns R., Brain J., Warner A. Gadolinium induces macrophage apoptosis. J. Leukocyte Biol. 1996; 59: 189–195
- Rhodes J. Macrophage heterogeneity in receptor activity: The activation of macrophage Fc receptor function in vivo and in vitro. J. Imm. 1975; 114: 975–981
- Wallace P., Keler T., Guyre P., Fanger M. Fc gamma R1 blockage and modulation for immunotherapy. Cancer Immunology 1997
- Wassef N., Alving C. Immunotherapy 1987; 45: 137–145
- Daeron M. Fc receptor biology. Ann. Rev. Imm. 1997; 15: 203–234
- Blanchette V., Kirby M., Turner C. Role of intravenous immunoglobin G in autoimmune hematologic disorders. Seminars in Hematology 1992; 29: 72–82
- Nydegger U., Sultan Y, Kazatchkine M. The conceopt of antiidiotypic regulation of selected autoimmune diseases by intravenous immunoglobins. Clin. Imm. Immunopath. 1989; 53: S72–82
- Wassef N., Alving C. Complement-dependent phagocytosis of liposomes by macrophages. Meth. Enzymology 1987; 149: 124–134
- Artursson P., Johansson D., Sjoholm I. Receptor-mediated uptake of starch and mannan microparticles by macrophages: relative contribution of receptors for complement, immunoglobins and carbohydrates. Biomaterials 1988; 88: 241–246
- Caron E., Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different rho GTPases. Science 1998; 282: 1717–1721
- Szebeni J., Fontana J., Wassef N., Mongan P., Morse D., Bobbins D., Stahl G., Bunger R., Alving C. Hemodynamic changes induced by liposomes and liposome-encapsulated hemoglobin in pigs: a model for pseudoallergic cardioplulmonary reactions to liposomes. Role of complement and inhibition by soluble CR1 and anti-C5a antibody. Circulation 1999; 99: 2302–2309
- Aderem A., Wright S., Silverstin Z., Cohn A. Ligated complement receptors do not activate the arachidonic acid cascade in resident peritoneal macrophages. J. Exp. Med. 1985; 161: 617–622
- Fadok V., Bratton D., Konowal A., Freed P., Westcott J., Henson P. Macrophages that have ingested apoptotic cells in vitro inhibit proinflamatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE-2 and PAF. J. Clin. Invest. 1998; 101: 890–898
- Peerschke E., Ghebrehiwet B. C1q augments platelet activation in response to aggregated Ig. J. Imm. 1997; 159: 5595–5598
- Blanchette V., Carcao M. Intravenous immunoglobin G and anti-D as therapeutic interventions in immune thrombocytopenic purpura. Transfusion Sci. 1998; 19: 279–288
- Debra M., Bonnet M., Fridman W., Carosella E., Philippe N., Reinert P., Vilmer E., Kaplan C., Teillaud J., Griscelli C. Infusion of Fc-gamma-fragments for the treatment of children with acute immune thrombocytopenic purpura. Lancet, 342: 945–949
- Bode A., Read M., Summaria L., Lust R. Lyophilized platelets; nearing clinical trials. Platelets 1997; 8: 436–437
- Bode A., Read M. Lyophilized platelets: Continued development. Transfusion Science 2000; 22: 99–105