Abstract
So far, perfluorocarbon (PFC) emulsions have been manufactured based mainly on two surfactants, Pluronic F-68 and egg yolk phospholipids (EYP) for clinical use. However, they have been documented to induce inflammatory or allergic responses when PFC emulsions were injected into human bloodstream. The cause of these side effects is associated with the phagocytosis of emulsified PFC microparticles by cells such as macrophages. In order to lessen the side effects, it is logic to develop surfactants, which are more phagocytosis-resistant and biocompatible. In this study, a perfluoroalkylated polyethylene glycol (RF-PEG) surfactant was synthesized by reacting perfluorooctanoyl chloride (C7F15COCl) with PEG of molecular weigh 8000. Both RF-PEG 8000 and EYP were used to make PFC emulsions separately by an ultrasonic homogenizer. Individual PFC emulsions were then incubated with mouse macrophage J774A.1 cells to examine the degree of phagocytosis. From microscopic observation of cell morphology, our results showed that the process of phagocytosis was retarded to a large extend using the RF-PEG surfactant. We also harnessed 19F-NMR to quantitatively detect the amount of PFC emulsions phagocytosed by J774A.1 cells. 19F-NMR result was consistent with the qualitative microscopic observation aforementioned.
INTRODUCTION
The idea of using PFC emulsions as submicron oxygen-carrying particulates for therapeutic purposes has been proposed for many decades Citation[1-3]. The endeavor for making PFC emulsions clinically feasible is reflected by mountains of articles. In 1989, U.S. FDA approval of Fluosol DA-20 (based on perfluorodecalin emulsions) as an oxygen transport agent set a major milestone in the history of medical use of PFCs Citation[[4]]. Although the use of this 1st generation of PFC emulsions was then ceased due to the issues such as low (20%) PFC content and requiring frozen storage, the development of 2nd generation of PFC emulsions (e.g., perflubron) has demonstrated remarkable improvement in maintaining stable PFC emulsions under room temperature for years Citation[[5]]. But some downsides are noticeable as well. PFC emulsions have been reported to associate with side effects (e.g., skin flushing and flu-like syndrome) after being injected into animal's bloodstream Citation[[6]]. The cause of these uncomfortable symptoms is related to the engulfment of PFC emulsions by the phagocytic cells of reticuloendothelial system Citation[[7]]. Since the surfactant (e.g., EYP and Pluronic F-68) used to emulsify PFC plays paramount roles on the process of opsonization which induces the ensuing phagocytosis, it is logic to develop surfactants, which are more phagocytosis-resistant, biocompatible, and can make intravenous half-life of the PFC particles extended longer. Unfortunately, among numerous investigations reported in the studies of PFC emulsions, there is a lack of design and synthesis of surfactants that can diminish phagocytic process.
In this study, perfluoroalkylated PEG 8000 (RF-PEG 8000) surfactant was synthesized to provide steric hindrance for decreasing phagocytosis of PFC emulsions. Both EYP and RF-PEG 8000 mediated PFC emulsions were incubated [chindividually with J774A.1 macrophages to examine the degree of phagocytosis. From microscopic observation of cell morphology, our results showed that, at the same incubation times, the degree of phagocytosis of the PFC droplets in the presence of RF-PEG 8000 surfactant was reduced to a large extent over that for the case of EYP. This qualitative observation was further validated by 19F-NMR spectra that were used to quantitatively determine the amount of PFC emulsions phagocytosed by macrophages. Finally, the hemolytic activity of this synthesized RF-PEG 8000 surfactant was confirmed by non-detectable hemolysis of red blood cells at even very high concentrations.
MATERIALS AND METHODS
Materials
Polyethylene glycol with molecular weight of 8000 (PEG 8000) was obtained from Union Carbide (Danbury, CT), pentadecafluorooctanoyl chloride (C7F15COCl) from Aldrich (Milwaukee, WI), and perfluorocarbon FC-40 from 3M (St. Paul, MN). Egg yolk phospholipid (EYP) was obtained from Sigma (St. Louis, MO). Other chemicals if not specified were purchased from Sigma. All of these materials were used as received without further purification.
Synthesis of Fluorinated PEG Surfactant
The perfluoroalkylated PEG 8000 surfactant (RF-PEG 8000) utilized in this study was synthesized by the esterification of PEG 8000 and C7F15COCl. A 250 ml two-necked round-bottomed flask equipped with a reflux condenser was immersed in a trough containing ice to assure the active ester reaction maintained at 4°C. First, 20 ml CH2Cl2 containing 0.005 mole of PEG 8000 was poured into the flask and mixed by a magnetic stirrer. In order to remove the hydrochloride generated from the ester reaction, 0.53 g (0.005 mole) of Na2CO3 were added in advance into the solution for absorbing HCl. Then, 2.17 g (0.005 mole) of C7F15COCl was added dropwise through a dropping funnel into the flask. To prevent any oxidization of reactants and products, nitrogen gas was injected into the reaction flask to purge out oxygen during the reaction. It took approximately one hour to have all of hydrophobic reagent C7F15COCl added gradually into the flask. After that, the reaction was continued for another 5 hours. The product mixture was filtered through a filter paper (Whatman, Ann Arbor, MI) to remove Na2CO3 particles. The filtered solution was transferred to a rotary evaporator heated at 60°C under vacuum to remove CH2Cl2. After cooling down to the room temperature, the white solid product left was the RF-PEG 8000 (i.e., C7F15COO-PEG 8000) surfactant.
Hemolysis Assay
Fresh human blood was drawn by venous puncture into heparinized tubes. Erythrocytes were separated by centrifugation at 1750 g for 10 min and resuspended to a hematocrit of 12.2% in PBS after washing with 10 mM PBS. 2 ml of the erythrocyte suspension and 2 ml of various concentrations of RF-PEG 8000 surfactant buffer solution were gently mixed in a test tube. The mixture was stored in a temperature-controlled water bath at 37°C with shaking. After 2 h, the mixture was immediately centrifuged at 1750 g for 10 min. The percentage of hemolysis was obtained from the absorbance (540 nm) of the supernatant containing the released hemoglobin. A 100% hemolysis stock lysate was prepared by dilution of the erythrocyte suspension with a 1.0 wt% aqueous solution of Triton X-100. A stable erythrocyte sample was prepared by the addition of the PBS solution to the erythrocyte suspension.
PFC Emulsification
The surfactant concentration used for making PFC emulsions was 2.5% (w/v) in water. Both FC-40 and surfactant solution were filtered through 0.2 μm syringe filters (Gelman, Ann Arbor, MI) within a biological safety laminar-flow hood. The PFC emulsions were obtained by homogenizing 1 ml of FC-40 and 2 ml of surfactant solution using an ultrasound generator (550 W, 20 kHz; Misonix, Farmingdale, NY) in the hood for 5 min under a programmed mode of 8 s on and 2 s off. Then, 6 μl of the PFC emulsion solution were added into 30 ml of Dulbecco's modified Eagle's medium (DMEM, Irvine Scientific, Santa Ana, CA) for the phagocytosis experiments described below. The size distribution of emulsified PFC particles was determined by a Malvern Mastersizer X-4700 laser light scattering equipment (Malvern Instrument Ltd., Worchester, UK).
Phagocytosis Experiments
The J774A.1 murine monocyte/macrophage cell line obtained from the American Type Culture Collection (ATCC, Rockville, MD) was selected for this study. A total of 2.5 × 107 cells were inoculated in a set of 15-cm Petri dishes (Falcon, San Jose, CA) with 30 ml of DMEM supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Rockville, MD). After 80% cell confluence, conditioned media were taken out and the dishes were rinsed three times with phosphate buffered saline (PBS, Gibco BRL). Then, freshly prepared PFC emulsions mixed with DMEM were vortexed and added on the top of the macrophages. The phagocytes then start to engulf the PFC emulsions made by EYP and RF-PEG 8000, respectively. After 9-h incubation at 37°C, the culture media were all replaced by 20 ml of PBS supplemented with 4 g of NaCl. With additional 3-min incubation, PBS solution containing cells and non-engulfed PFC emulsions were collected into centrifuge tubes. The tubes were centrifuged at 1280 g for 3 min. After centrifugation, the supernatants containing non-engulfed PFC emulsions were removed and cells were re-suspended with 20 ml of fresh PBS solution. Same centrifugal procedure was repeated two more times to wash out extracellular PFC emulsions thoroughly. In the end, 3 ml of fresh PBS solution were used to re-suspended cells. One ml of the resulting cell suspension was directly transferred to the 5-mm calibrated NMR tube for 19F-NMR detection. Cell number was numerated by a hemocytometer. The microscopic observation of the cell morphology associated with PFC emulsions manufactured by the two surfactants was photographed. All cultures were done at 37°C and balanced with 5% CO2 in a 100% humidified incubator.
19F-NMR Studies
Sodium trifluoromethane sulfonate (CF3SO3Na; 0.08 g) employed as the internal NMR standard was added to the aforementioned NMR tube containing PFC-laden macrophages. After gentle mixing of CF3SO3Na with cells, N2 gas was used to purge out the O2 gas dissolved in the cell-containing medium. The NMR tube was spun for 1 min and the 19F-NMR spectrum was recorded on a Bruker DRX-400 Avance (Bruker Instruments Inc., Billerica, MA) spectrometer operated at 400 MHz using a dedicated 19F probe. Standard acquisition parameters inclu[chded temperature at 27°C, spectral width of 37665 Hz, acquisition time of 0.436 s, and fidres of 1.147 Hz. The spectrum data were analyzed by integrating the surface areas covered by the terminal CF3 signals of the internal standard and of the FC-40 engulfed by cells. The amount of FC-40 emulsions ingested by J774A.1 cells was hence determined.
RESULTS AND DISCUSSION
The synthesized RF-PEG 8000 surfactant with a yield of 96% was used directly for the development of FC-40 emulsions without further purification because any existing impure components without the ability to make the emulsions were removed by centrifugation. The mean particle size of FC-40 emulsions made by EYP and RF-PEG 8000 was calculated to be 0.32 μm and 0.58 μm, respectively. Prior to the phagocytosis experiments, the stability of PFC emulsions created by RF-PEG 8000 needs to be examined. This is because the ester linkage of RF-PEG 8000 surfactant was prone to be hydrolyzed in aqueous solution. Since the breakage of ester bond increases the pH value of the fluorosurfactant-containing water solution, the degree of hydrolysis of RF-PEG 8000 can thus be indirectly determined by the change of pH value. The time course profile of pH variations for C7F15COO-PEG 8000 dissolved in water under 37°C was determined (data not shown). It showed that the drastic decrease of pH value occurred around 12 h. In order to avoid interference by the instability of PFC emulsions resulted from hydrolysis, our phagocytosis experiments were therefore performed within 9 h. To extend the time of hydrolytic endurance for future study, perfluoroalkylated PEG surfactants with stable amide or urethane linkage need to be developed. The hemolytic activity of this synthesized fluorosurfactant was determined to be non-detectable hemolysis of erythrocytes up to very high concentration of 0.5% w/v (data not shown). This outcome was consistent with other group's study demonstrating that hemolytic activity on human red blood cells was negligible even at very high concentrations of fluorocarbon-tailed derivatives Citation[[8]].
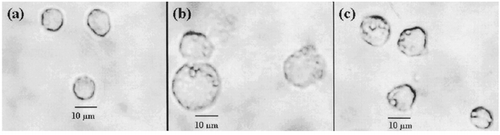
Cell morphology was observed and photographed under an inverted light microscope to qualitatively determine the role surfactants play on the degree of PFC emulsions engulfed by J774A.1 cells. Generally speaking, the increment of cell size is proportional to the amount of PFC emulsions engulfed. illustrated that after 9-h incubation with two different surfactants mediated PFC emulsions, cells exhibited different degree of augment in size. The average size of non-PFC emulsions treated normal cells was around 9 μm in diameter. Cell size increased to approximately 11 and 17 μm in diameter after 9-h incubation with FC-40 emulsions manufactured by RF-PEG 8000 and EYP, respectively. It is obvious that the capability of RF-PEG 8000 to prevent FC-40 emulsions from the phagocytosis of J774A.1 cells is superior to that of EYP. This probably is due to the long PEG chain length provides steric hindrance and less protein adsorption to emulsified PFC microparticles Citation[[9]]. It is expected that PEG-based surfactants applied to the PFC emulsions would exclude large molecules such as plasma proteins but would not interfere with the diffusion of small molecules (e.g., respiratory gases) across the PEG barrier.
Figure 1. Morphological changes of J774A.1 macrophage cells after the engulfment of PFC emulsions manufactured by (b) EYP and (c) RF-PEG 8000, respectively. The cells shown in (a) were the normal cells without the treatment of PFC emulsions. Compared with EYP-mediated PFC emulsions, the degree of phagocytosis was ameliorated to a large extent for RF-PEG 8000 mediated ones. Photomicrographs were taken after 9-h incubation (Magnification × 100).
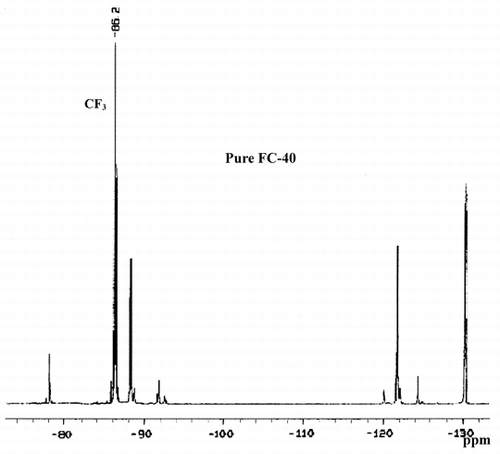
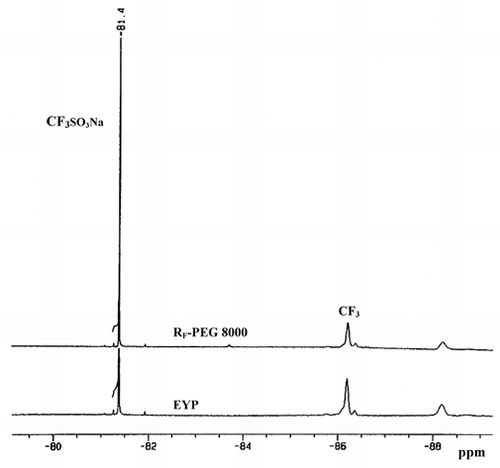
Although the phagocytosis of PFC emulsions can be determined by microscopic observation, this tedious and qualitative approach cannot provide an accurate phagocytic rate. A quantitative approach using 19F-NMR was thus developed. showed the 19F-NMR spectrum of pure FC-40. The absorption peak of CF3 group located at about −86.2 ppm was harnessed to determine the amount of FC-40 emulsions phagocytosed by the J774A.1 cells. presented 19F-NMR spectra of fluorine contained within the cells that phagocytosed emulsified PFC microparticles made by two different surfactants after 9-h incubation. The degree of phagocytosis obtained by quantitative 19F-NMR was coincided with the microscopic observation of cell size change mentioned previously. It should be noted that there is no major chemical shift of the CF3 absorption peak even though the FC-40 emulsions were within the cells. In addition, since the concentration of fluorosurfactant RF-PEG 8000 used is relatively low, its influence to the CF3 peak is probably negligible. In general, the measurement indicated that the degree of phagocytosis of the PFC microparticles in the presence of RF-PEG 8000 surfactant was reduced about 2-fold over that for the case of EYP.
ACKNOWLEDGMENTS
We are indebted to Dr. Herbert Meiselman's group for the support of hemolysis assay. Thanks also go to Chang-Chin Kwan and Yuli Wang for their valuable technical assistance.
REFERENCES
- Clark L. C., Gollan F. Survival of mammals breathing organic liquids equilibrated with oxygen at atmospheric pressure. Science 1966; 152: 1755–1756
- Yokoyama K., Yamanouchi K., Watanabe M., Matsumoto T., Murashima R., Daimoto T., Hamano T., Okamoto H., Suyama T., Watanabe R., Naito R. Preparation of perfluorodecalin emulsion, an approach to the red cells substitute. Fed. Proc. 1975; 34: 1748–1483
- Geyer R. P. Bloodless” rats through the use of artificial blood substitutes. Fed. Proc. 1975; 34: 1499–1505
- Lowe K. C. Perfluorochemicals in vascular medicine. Vascular Med. Rev. 1994; 5: 15–32
- Dietz N. M., Joyner M. J., Warner M. A. Blood substitutes: fluids, drugs, or miracle solutions?. Anesth. Analg. 1996; 82: 390–405
- Flaim S. F., Hazard D. R., Hogan J., Peters R. M. Characterization and mechanism of side-effects of Oxygent HT (high concentration fluorocarbon emulsion) in swine. Art. Cells, Blood Subs., and Immob. Biotech. 1994; 22: 1511–5
- Flaim S. F. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Art. Cells, Blood Subs., and Immob. Biotech. 1994; 22: 1043–1054
- Zarif L., Riess J. G., Pucci B., Pavia A. A. Biocompatibility of alkyl and perfluoroalkyl telomeric surfactants derived from THAM. Art. Cells, Blood Subs., and Immob. Biotech. 1993; 21: 597–608
- Harris J. M. Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications. Plenum Press, New York 1992; 1–14