INTRODUCTION
Hypercholesterolemia has been identified as a major risk in the development of premature atherosclerosis and in its clinical sequelae is similar to coronary artery disease and myocardial infarction. It is generally agreed that low-density lipoprotein (LDL) cholesterol levels above 130 mg/dl are associated with a very high incidence of coronary heart disease (Citation[[1]]). Recently scientists found that drastic reductions of LDL by diet, exercise, and drugs can stop the progression of coronary stenoses or even induce regression of atherosclerotic lesions (Citation[[2]]). However, a sufficient reduction of LDL-cholesterol can not be attained by conservative treatment alone in many instances, such as familiar hypercholesterolemia, and further extracorporeal LDL removal by plasmapheresis is necessary. Studies on LDL apheresis has been carried out by unselective plasma exchange (Citation[[3]]), membrane filtration (Citation[[4]], Citation[[5]]), chemoadsorption by dextran sulfate (Citation[[6]]), immunoadsorption (Citation[[7]]) with immobilized anti-LDL-antibodies, and heparin-induced extracorporeal LDL precipitation (Citation[[8]]). Apheresis in clinical treatments is often performed in condition with adsorbents, including sulfonated dextran, sulfonated polyvinyl alcohol, polyacrylate, heparin, and anti-LDL antibodies. Cholesterol is known to act as a carrier for the transporting of LDL in human bodies (Citation[[9]]). According to this natural phenomenon, the author developed a new adsorbent by immobilizing cholesterol modified dextran on agar beads for the removal of LDL from serum of hypercholesterolemia patients.
MATERIAL AND METHODS
Chemicals
Cholesterol: biotechnological reagent was purchased from Tianjin East Material Factory; dextran: biotechnological reagent from Tianjin Hemotology Research Institute and 1,6-hexyldiisocyanate of analytical reagent from F.K. Company. All other chemicals were analytical reagents and purchased from Tianjin reagent company. Serum from hypercholesterolemia patients was supplied by Tianjin 3rd Central Hospital. Agar beads and cholesterol modified dextran were tailor-made at Bioactive Material Research Laboratory of Nankai University, Tianjin, China.
Preparation of the Ligand
The preparation of ligand was carried out in two steps: (1) the synthesis of cholesterol modified dextran and (2) the introduction of the amino group on the dextran molecule, followed by linking to the agar beads by glutaraldehyde.
Synthesis of Cholesterol-Modified Dextran
Cholesterol (7.8 g) was reacted with 1,6-hexyldiisocyanate 48 ml in 200 ml dry toluene at 80° for 48 hours. Pyridine (4 ml) was added to the solution as catalyst in the beginning of the reaction. After the solvent was removed, 600 ml petroleum ether (60–90°C) was added to the residue and it was stored overnight at −10°C.The precipitate obtained was separated and dried in a vacuum to give cholesterol N-(6-isocyanohexyl) carbamate as a white powder; yield-3.58 g.
Five hundred milligrams of cholesterol N-(6-isocyanohexyl) carbamate were reacted with 4.00 g dextran Mw70000 in 100 ml dry DMSO at 80° for 8 hours. Pyridine (8 ml) was added as a catalyst to the solution in the beginning of the reaction. Ethanol (500 ml) was added to the reaction mixture and the suspension so obtained was stored overnight at 4°C. The precipitate was separated, purified by dialysis against water for 24 hours, and dried in a vacuum to give a white powder (3.78 g) of cholesterol modified dextran. The degree of substitution of the cholesterol group was determined by elemental analysis to be 4 cholesterol groups in 100 dextran glucose units.
Preparation of Aminoethylamidosuccinate Dextran (Citation[[10]])
One gram of cholesterol-modified dextran in 5 ml DMSO was reacted with dry pyridine and 0.3 g succinic anhydride in a reaction vessel. The solution was stirred for 18 hr at 40°C then, 100 ml ethanol was added to the reaction mixture and the precipitate was separated, purified by dialysis against water using a seamless cellulose tube, and lyophilized to give succinate dextran as a white powder; The degree of substitution of COOH was 4.7 COOH groups per 100 glucose units.
This 1.48 ml of 50% aqueous ethylenediamine, 2 ml of water and 5 ml of pyridine were added to a cooled solution (4°C) of 2 g succinate dextran in 6 ml of water. 1.91 g of dicyclohexylcarbodiimde (DCC) in 5 ml pyridine was added to the stirred solution, and the reaction mixture was stirred for 48 h at 20°C. 100 ml of ethanol was added to the reaction mixture and the precipitate was separated, purified by dialysis against water using a seamless cellulose tube, and lyophilized to give a white powder of aminoethylamidosuccinate dextran; found: N=1.83%.
Preparation of Cholesterol Modified Dextran Adsorbent
Aminoethylamidosuccinate dextran (15 mg) was dissolved in 1.6 ml sodium carbonate buffer (PH9.5) containing 0.4 ml DMSO. One milliliter of epoxy-activated 10% agar beads was added to the solution. The reaction mixture was shaken for 24 h at 40°C. The agar beads were then thoroughly washed with 100 ml of water. The concentration of dextran derivative was determined by the phenol-sulfuric acid method (Citation[[11]]).
Determination of Adsorption Capacity of the Adsorbent
One milliliter adsorbent was mixed with 3 ml hypercholesterol serum and shaken for 2 h at 37°C. The total cholesterol (TC), total triglycerides (TG), and LDL and HDL levels were determined by analytical kits (Citation[[12]]).
Determination of Nonspecific Adsorption Capacity of Adsorbent
One milliliter adsorbent was mixed with 3 ml hypercholesterol serum and shaken for 2 h at 37°C. The albumin and immunoglobulin levels were determined by analytical kids.
Heat-Stability Experiment
One milliliter wet-state adsorbent was mixed with 5 ml NaCl solution (0.9%) and heated for 30 min at 125°C in an autoclave. After steam sterilization, the ligand leakage and adsorption efficiency were determined.
RESULTS AND DISCUSSIONS
Adsorption Capacity of LDL Adsorbent
The cholesterol-modified dextran-agar bead type adsorbent has good mechanical properties with smooth surface and was packed in a shunt for the removal of LDL. The adsorption capacities of LDL and cholesterol are shown in STable .
Table 1. Amount of Ligand Attached on Carrier vs. Its Adsorption Capacity (The Initial Concentrations of TC, TG, LDL-C, and HDL-C Were 248.6, 167, 183, and 57 mg/dl, Respectively)
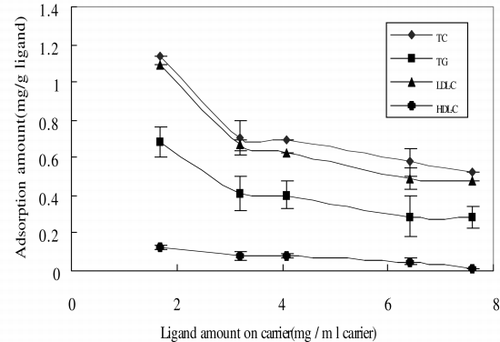
The adsorption capacity of LDL and cholesterol in serum increases with the increased amount of ligand attached on agar beads. At 7.6 mg/ml, the adsorption capacities for TC, TG and LDL levels were 53.9%, 43.0%, and 65.8%, respectively. It is interesting to note that the adsorption capacity of HDL also increases with the increased amount of ligand attached to agar beads, the highest percentage of HDL was 20.3%. Compared to the LDL adsorption efficacy (65.8%), it is evident that the adsorbent has a higher selectivity for LDL than HDL.
shows the adsorption efficacy per gram ligand decreased with the increasing amount of ligand attached to the agar beads. The reason for this decreasing efficacy may be due to the steric effect of the large LDL molecule (2000 KD).
Figure 1. Adsorption amount vs. the amount of ligand. The initial concentrations of TC, TG, LDL-C, and HDL-C were 296.7, 167, 183, and 48 mg/dl, respectively.
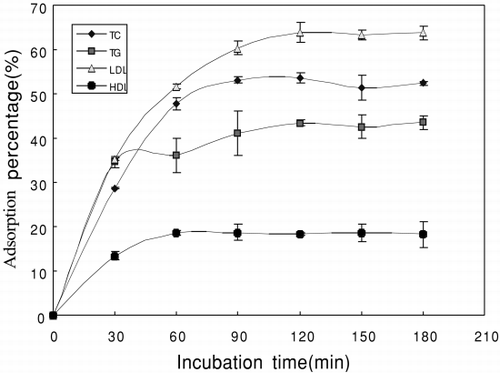
Experimental results in show the adsorption percentage of adsorbent increases with prolonged incubation time and reach equilibrium after 120 min.
Nonspecific Adsorption of the Adsorbent
The nonspecific adsorption of the new adsorbent was tested by adsorption of albumin and total immunoglobulin in vitro experiments (see ).
Table 2. Amount of Ligand Attached vs. Nonspecific Adsorption of Adsorbent
The albumin and total immunoglobulin levels before and after the adsorption of the adsorbent are presented in . Results show the adsorption capacity of albumin is less than 5% and total immunoglobulin protein is less than 10%, indicating the adsorbent has good selectivity.
Heat-Stability Properties of the Adsorbent
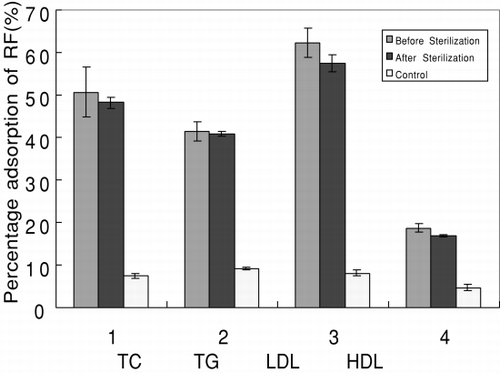
Heat-stability properties of the adsorbent were studied during steam-sterilization. No significant leakage of the ligand was observed after steam sterilization in .
Table 3. The Leakage of Ligand After Steam Sterilization
The covalent bond between the ligand and the carrier is responsible for the good heat stability property of the adsorbent.
shows that high temperature during sterilization did not obviously decrease the adsorption efficiency of the adsorbent. Therefore the traditional autoclave can be used to sterilize the adsorbent.
Figure 3. The difference in adsorption capacity of the adsorbent before and after steam sterilization. The initial concentrations of TC, TG, LDL-C, and HDL-C were 234.8, 123, 176, and 47.3 mg/dl, respectively.
In conclusion, the novel LDL adsorbent has a high potential application for the removal of LDL in hemoperfusion.
ACKNOWLEDGMENTS
The support of the National Key Project of Fundamental Research and Advances (G1999064707) is gratefully acknowledged.
REFERENCES
- Pekkanen J., Linn S., Heiss G., Suchindran C., Leon A. Ten year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N. Engl. J. Med. 1990; 322: 1700–1707
- Thompson G. R. Progression and regression of coronary artery disease. Curr. Opin. Lipodol. 1992; 3: 263–267
- Thompson G. R. Plasma exchange for hypercholesterolemia. Lancet 1981; 1: 1246–1248
- Mabuchi H., Michishita I., Sakai T. Treatment of homozygous patients with familial hypercholesterolemia by double filtration plasmapheresis. Atherosclerosis 1986; 61: 135–140
- Soltys P. J., Etzel M. R. In vitro characterization of a membrane-based low-density lipoprotein affinity adsorption device. Blood Purif. 1998; 16: 123–134
- Yokoyama S., Hayashi R., Satani M., Yamamoto A. Selective removal of LDL by plasmapheresis in familial hypercholesterolemia. Atheriosclerosis 1985; 5: 613–622
- Koll R. LDL-therasorb immunoadsorption for the treatment of severe hypercholesterolemia refractory to conventional therapy. Ther. Apheresis 1998; 2: 142–146
- Wieland H., Seidel D. A. Simple specific method for precipitation of LDL. J. Lipid Res. 1983; 24: 904–909
- George M., Whitesides J. P., Mathias C. T. A receptor-mediated pathway for cholesterol homeostasis. Science 1991; 254: 1312–1319
- Klyashchitsk B. A., Mitina V. Kh. Affinity adsorbents with polysaccharide spacers preparation and properties. J. Chromatogr. 1981; 210: 55–65
- Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956; 28: 350–355
- Cui F. Medical Biochemistry Analysis Handbook. Tianjin Science and Technology Publishing Company. 1981; 224