Abstract
As the blood substitute Diaspirin Cross-linked Hemoglobin (DCLHb) has potent vasopressor activity, we assessed its hemodynamic effects in a clinically relevant dopamine-resistant endotoxic shock model in swine. In a randomized and controlled study, E. coli LPS was administered to anesthetized and invasively monitored swine. Group I (n=3) control pigs were not resuscitated. Groups II (n=5) and III (n=6) pigs received dopamine (DA) after MAP decreased 30%, and hetastarch and DCLHb, respectively, after dopamine-resistance occurred. Progressive hemodynamic decline occurred in Group I pigs. DA failed to restore MAP to baseline. However, 0% and 67% of pigs also treated with hetastarch and DCLHb, respectively, achieved temporary restoration of baseline MAP (p=0.03), prompting a reduction in the dose of DA in 0% of hetastarch vs. 50% of DCLHb treated pigs. Except for increased MPAP and decreased heart in DCLHb treated pigs (p<0.001), hemodynamics and survival were not different (p>0.05). In conclusion, although DCLHb exacerbated pulmonary hypertension and did not improve O2 utilization or survival, because DCLHb restored MAP to baseline and had a dopamine sparing effect, further investigation of DCLHb's hemodynamic effects in adrenergic agent-resistant endotoxemia is warranted.
INTRODUCTION
Half of septic deaths are associated with refractory hypotension.Citation[[1]] The etiology of vascular unresponsiveness to adrenergic agents in advanced sepsis (SEP) is multi-factorial. However, activation of inducible nitric oxide synthase (iNOS) and consequent vasodilation from excessive nitric oxide (NO) production play a notable role.Citation[[2]] Hemoglobin-based O 2 carriers (HBOCs) reverse septic hypotension in vivo and adrenergic unresponsiveness in vitro, mainly by binding NO.Citation[[3]], Citation[[4]] Furthermore, HBOCs improve O 2 delivery in septic animal modelsCitation[[5]] and perfuse small capillary beds not normally traversed by red blood cells.Citation[[6]] Therefore, we hypothesized that DCLHb could increase O 2 delivery, O 2 uptake, and survival in a dopamine-resistant (DR) endotoxic shock animal model designed to simulate the clinical scenario of vasopressor-resistant septic shock that is commonly seen in Intensive Care Unit patients.
MATERIALS AND METHODS
All studies were conducted with approval of University of Vermont's Institutional Animal Care and Use Committee. Anesthesia was induced and maintained with intramuscular ketamine (20 mg/kg) and halothane/O 2 mixture (1.2/98.8%), respectively. Pigs were intubated. The femoral artery was catheterized for BP monitoring and blood sampling, and an introducer and pulmonary artery catheter were placed in the femoral vein (Arrow, Reading, PA).
E. coli LPS (O26:B6) 75 μg/kg was administered over 10 minutes followed by a 125 μg/kg/hour infusion. These doses were chosen after a dose-escalation trial with 5 pigs demonstrated that these doses induced gradual progressive dopamine-resistant shock.
Pigs were randomly allocated to 3 groups: Group I (n=3) received LPS alone without dopamine (DA) resuscitation; Group II (n=5) received LPS, DA resuscitation, and hetastarch (HS) salvage; Group III (n=6) received LPS, DA resuscitation, and DCLHb salvage. In Groups II and III pigs, after developing SEP (arbitrarily defined as a 30% decrease in MAP below baseline [BL]), DA was infused at 5 μg/kg/min, and titrated upward by 5 μg/kg/min every 10 minutes to 20 μg/kg/min. If MAP did not reach BL despite the maximum dose of DA, “dopamine resistance” (DR) was defined (new time 0). Group II pigs were then given 250 ml of HS over 1 hour. Group III pigs were then treated with DCLHb at 5 mg/kg/min, titrated upward every 5 minutes to 15 mg/kg/min to a total volume of 250 ml. This volume load approximated the 20 ml/kg crystalloid fluid bolus that is common in clinical practice. If restoration of BL MAP occurred, the DA dose was titrated downward. Hemodynamics and blood assays were followed for up to 6 hours. In order to calculate DCLHb's contribution to O 2 content, free hemoglobin levels were measured in Group III pigs.
Total nitrate intermediates (TNI) were measured in Group II and III pigs according to the manufacturer's instructions (Nitrate/nitrite Colorimetric Assay Kit, Cayman Chem., San Diego, CA). Plasma endotoxin levels were quantified on 4 randomly chosen pigs prior to salvage drug administration by the Pyrotell Limulus amebocyte lysate single test gel-clot method according to the manufacturer's instructions (Pyrotell LAL gel-clot assay, Associates of Cape Cod, Cape Cod, MA). The standard series demonstrated an endotoxin sensitivity of 0.025 ng/ml.
Statistics
For numerical data, results of repeated measures ANOVA comparing group means are reported; significant interactions were evaluated by the Student–Newman–Keuls test. A binomial test compared MAP, HR, and MPAP curves. Fisher's exact test and the Mantel–Cox test were used to compare the proportions of pigs restoring MAP to BL in Groups II and III, and survival analysis, respectively.
RESULTS
Sepsis (SEP)
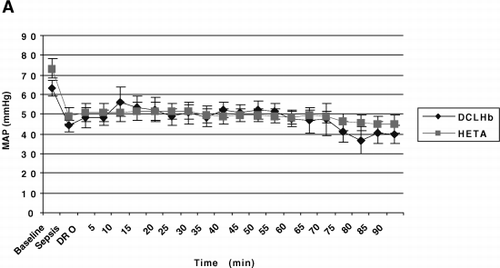
LPS infusion caused at least a 30% decline in MAP in all 3 groups, defining SEP in 75–90 minutes (p<0.001) (A). For Groups II and III, the combined mean systemic vascular resistance index (SVRI) trended downward from 1280 dyne×sec/cm 5/M 2 at BL to 989 dyne×sec/cm 5/M 2 at SEP (−23%) (p=0.07). Base excess decreased from +3.3 to −0.32 mM/L (−110%) (p<0.05). MPAP increased from 23 to 28 mm Hg (+19%) (p<0.05). From BL to SEP, cardiac index (CI) (3.8 to 3.4 L/min/M 2), stroke volume index (SVI) (35 to 32 ml/beat/M 2), heart rate (HR) (110 to 112 beats/min), and calculated O 2 delivery (417 to 458 L/min/M 2) and O 2 uptake (78 to 90 ml/min/M 2) did not change significantly (p>0.05).
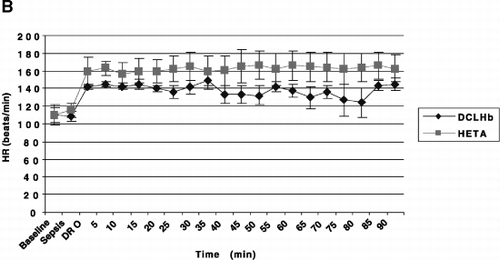
Figure 1. The hemodynamic effects of endotoxemia in swine with and without dopamine (DA) resuscitation and DCLHb and hetastarch (HETA) salvage. Pigs were administered LPS. Group I pigs received no treatment. In treatment Groups II and III, DA was administered when “sepsis” developed (≥30% decline in MAP). When “dopamine resistance” occurred (20 μg/kg/min failed to restore MAP to baseline), hetastarch (Group II) and DCLHb (Group III) were added. Time 0=pre-LPS infusion. Values are mean±SEM. Only comparisons of CI were significantly different (p≤0.05).
Untreated Control Pigs
In order to demonstrate DA's efficacy, and the applicability of our study model, Group I pigs were not resuscitated with DA. These pigs exhibited a trend toward more severe hypotension (p=0.08) and less severe pulmonary hypertension (p=0.07), as well as depressed CI (p=0.01) and early mortality (p=0.03) ().
Dopamine (DA) Resuscitation
Although DA resuscitation arrested the fall in MAP in Group II and III pigs, restoration of BL MAP did not occur—defining DR (A). DA infusion (from SEP to DR), increased combined mean HR 34% (112 to 151 beats/min, B), resulting in 32% and 47% increases in CI (3.4 to 4.5 L/min/M 2, D) and DO 2 (458 to 675 L/min/M 2), respectively (p<0.05). The O 2 extraction ratio (O 2ER) trended downward (20% to 13%) (p>0.05), and VO 2 was stable (90 to 85 ml/min/M 2) (p>0.05). DA slowed deterioration of lactic acidosis and base deficit. As SVI remained stable (32 to 31 ml/beat/M 2) (p>0.05), the increased CI was predominantly a consequence of tachycardia. SVRI trended downward 26% (989 to 730 dyne×sec/cm 5/M 2) (p>0.05), and PO 2 trended downward 25% (441 to 330 mm Hg) despite DA infusion (p>0.05). DA increased MPAP 23% (28 to 34 mm Hg, C) (p<0.05). For the two treatment groups, no difference was seen for these parameters (p>0.05).
Salvage Drugs
From 30 to 60 minutes after starting DCLHb and HS, MAP did not change significantly (51/50/49 mm Hg for Group II, and 48/49/48 mm Hg for Group III, at DR/30/60 minutes, respectively, A) (p>0.05). However, the interim vasopressor response to DCLHb was profound—restoration of BL MAP was achieved in 0% of Group II vs. 67% of Group III pigs (p=0.03). DA was titrated downward (temporarily) in 0% and 50% of Group II and III pigs, respectively.
DCLHb dampened DA'S chronotropic effect. From DR to 60 minutes after starting salvage drugs, heart rates did not change significantly (160 to 166 beats/min, and 143 to 137 beats/min, in Groups II and III pigs, respectively) (p>0.05). Nevertheless, the DCLHb curve was significantly lower than the hetastarch curve (p<0.001) (B).
A bimodal pattern was noted for MPAP. First, a brief rise in MPAP was noted immediately post-LPS infusion in all 3 groups-combined MPAP increased 43% by 15 minutes (23 to 33 mm Hg) (p<0.05). This was followed by a prolonged rise with a plateau in the DA treated groups (C and C) that was significantly greater only in a pair-wise comparison of Group I and III (p=0.03). LPS infusion led to the following changes in MPAP by 90 minutes: −9%/+27%/+29% in Groups I/II/III, respectively. Upon infusion of salvage drugs (from DR to 90 minutes), MPAP increased 10% (34 to 38 mm Hg) and 27% (35 to 44 mm Hg), respectively (p>0.05). However, the DCLHb curve was significantly higher than the hetastarch curve (p<0.001).
CI and DO 2 fell in both groups. Sixty minutes after starting salvage drugs, CI decreased from 3.9 to 2.8 L/min/M 2 (−29%) (p<0.05) and 4.9 to 2.1 L/min/M 2 (−57%) (p<0.05), in Groups II and III, respectively (p=0.06) (D). Calculated DO 2 decreased from 577 to 438 ml/min/M 2 (−24%) (p<0.05) and 740 to 361 ml/min/M 2 (−51%) (p<0.05), respectively (p=0.20).
In both treatment groups, VO 2 was maintained for at least 120 minutes by a temporary compensatory increase in O 2 extraction. From DR to 60 minutes after starting salvage drugs, O 2ER increased from 13 to 25% (p<0.05) and 12 to 35% (p<0.05), in Groups II and III, respectively (p=0.14). VO 2 was 76 ml/min/M 2 and 108 ml/min/M 2 (p>0.05), and 92 ml/min/M 2 and 110 ml/min/M 2 (p>0.05), at DR and 60 minutes, respectively (p=0.6).
As lactic acidosis and base deficit worsened to an equal degree in both groups (p>0.05), no differences in the adequacy of VO 2 could be documented for the metabolic demand of the 2 groups. From DR to 60 minutes after starting salvage drugs, BL decreased from −1.7 to −3.9 mM/L (−129%) (p<0.05) and −1.8 to −4.7 mM/L (−160%) (p<0.05), in Groups II and III, respectively (p=0.61); PO 2 did not change significantly: 268 to 238 mm Hg (−11%) (p>0.05) and 371 to 300 mm Hg (−19%) (p>0.05), respectively (on 98.8% O 2). There were no differences between PO 2 values for Groups II and III (p=0.96).
Total Nitrate Intermediates (TNI)
TNI were not statistically different at BL, SEP, or DR—2.0 μM/3.6 μM/3.5 μM, and 3.6 μM/1.2 μM/1.9 μM, for DCLHb and HS treated pigs, respectively. Two samples were measured in DCLHb treated pigs upon restoration of MAP to BL. A 77% decrease was seen in these samples (to 0.8 μM).
Endotoxin and Survival
All four BL test samples were negative for endotoxin. At 150 minutes after LPS infusion, all were positive at dilutions equivalent to 25.6–51.2 ng/ml of endotoxin.
No significant changes in methemoglobin were noted (data not shown). Six hours after BL, survival was 0% in Group I, 40% in Group II, and 17% in Group III (p=0.03)—Group I vs. II (p=0.02), Group I vs. III (p=0.10), and Group II vs. III (p=0.20).
DISCUSSION
In the lethal DA-resistant endotoxic swine model described, DCLHb demonstrated a profound yet ephemeral systemic vasopressor response. Sixty-seven percent of DCLHb pigs achieved BL MAP vs. 0% of hetastarch treated pigs, prompting a reduction in the dose of DA only in DCLHb treated pigs. However, DCLHb exacerbated endotoxin- and DA-related pulmonary hypertensive changes. Other hemodynamic parameters were essentially the same in DCLHb and hetastarch treated pigs. Moreover, DCLHb did not improve O 2 utilization or survival. Our data suggest a possible role for use of DCLHb as a vasopressor agent in endotoxic shock; however, addition of agents known to decrease pulmonary hypertension may be helpful (e.g., non-steroidal anti-inflammatory drugs [NSAIDs] and/or inhaled NO). A larger trial powered to detect hemodynamic and survival differences that were not documented in this pilot study is needed.
Why was a generalized hemodynamic benefit not seen in the DCLHb treated pigs? First, DA dosing was decreased only in DCLHb treated pigs; it is possible that addition of DCLHb, rather than substitution of DCLHb, would have resulted in more significant benefits. Second, coincidentally, at the start of salvage therapies, PCWP was significantly higher in the hetastarch group than in the DCLHb group (data not shown). Third, more severe pulmonary hypertension in DCLHb pigs may have compromised cardiac function. As has been described in endotoxemic swine, we noted a bimodal pattern of pulmonary hypertension. The brief initial effect is related to release of arachidonic acid metabolites, as it is reversed by NSAIDs. The latter more persistent effect is at least in part a consequence of NO binding, as it is attenuated by inhaled NO.Citation[7-11] Fourth, could DCLHb have resulted in excessive vasopressor activity and cardiovascular compromise, as occurs with NOS inhibitors?Citation[12-14] The fact that indices of cardiac function decreased (i.e., CI and SVI) and SVRI increased similarly, in both treatment groups, argues against these explanations; however, contractility was not measured directly.
Fifth, HBOC-related enhancement of endotoxin bioavailability and post-resuscitation re-perfusion injury with liberation of toxic free radicals have been described;Citation[15-18] neither were evaluated in this study. Sixth, in our lethal swine model, CI and SVR remained depressed despite maximum doses of DA, so an advanced hypodynamic state of irreversible decompensation had already occurred. That may have precluded any intervention from documenting a benefit without a very large study, or a larger dose of DCLHb, or addition of rather than substitution of DCLHb. However, we demonstrated post-LPS infusion endotoxin levels to be 25–51 ng/ml in our study. As similar endotoxin levels were reported in septic ICU patients,Citation[[19]], Citation[[20]] our model may simulate clinical end-stage sepsis well. Finally, the consequences of NO binding must be considered.
NO and Sepsis
Since Furchgott and Zawadski's initial description of endothelial derived relaxation factor (EDRF) and Palmer and Ignarro's identification of NO's EDRF activity,Citation[21-23] a number of investigators have demonstrated that excessive NO production is key to adrenergic agent vascular unresponsiveness in SEP.Citation[[2]], Citation[[24]] Similar to free hemoglobin, DCLHb's ferrous group presumably binds NO directly, preventing NO from binding the ferrous group of vascular smooth muscle guanyl cyclase, and thereby causing vasoconstriction. Whether HBOCs are allosterically S-nitrosylated at the recently described hemoglobin reactive cysteine SH sites is unclear.Citation[[25]]
NO Inhibition in Sepsis
In light of the multiple effects of NO, it is not surprising that studies of NO inhibition in SEP have had mixed results. Inhibition of NO has been approached from a number of angles: L-arginine analogue nonspecific NO synthase inhibitors (NOSI); inducible NOS (iNOS) specific inhibitors; attempts to inhibit NOS in selected systemic vascular beds; and HBOCs that have no effect on NOS activity but bind NO directly. Studies of NOSI in SEP in animals and humans have demonstrated reversal of hypotension even in adrenergic resistant models. However, some investigators have shown that high doses can cause cardiovascular collapse secondary to excessive systemic and pulmonary vasoconstriction.Citation[26-32]
HBOCS in Sepsis
In animal studies of HBOCs in SEP, reversal of the hyperdynamic state via increased vascular resistance and blood pressure, and either unchanged or decreased CO, have been shown; however, exacerbation of endotoxin-related pulmonary hypertension (at least partially by decreasing NO) has been almost uniformly reported.Citation[33-35] Mourelatos showed improved survival in septic rats administered DCLHb, but only if administered within 2 hours of induction of SEP.Citation[[36]] They argued that too early, DCLHb exacerbates pulmonary hypertension, and too late, irreversible decompensation will have occurred. Krishnamurti reported that concomitant administration of LPS and an HBOC markedly increased mortality and cardiovascular toxicity in rabbits. Supporting Mourlelatos' data, mortality was reduced by delaying infusion of the HBOC until 4 hours after LPS infusion.Citation[[37]] In one human trial, a significant reduction in vasopressor requirement was documented in critically ill ICU patients (mostly sepsis and SIRS) administered DCLHb.Citation[[38]] Finally, studying isolated aortic ring preparations, HBOCs have been shown to reverse vascular unresponsiveness to adrenergic agents in vitro at least partially secondary to NO binding.Citation[[4]], Citation[[39]]
Although increased TNI have been reported in septic shock models,Citation[[40]] we did not find a consistent increase. However, consistent with Bone's results studying pyridoxalated hemoglobin in ovine SEP,Citation[[34]] a trend toward decreased TNI after DCLHb administration was noted in our study as well—presumably, secondary to NO binding by DCLHb.
In addition to reversing hypotension in adrenergic unresponsive endotoxemia, a specific aim of this study was to demonstrate improved O 2 delivery and uptake, because these parameters have been correlated with survival.Citation[[41]], Citation[[42]] Sielenkamper reported improved VO 2 after DCLHb administration in O 2 delivery dependent septic rats.Citation[[5]] Additionally, impaired red blood cell deformability has been reported in SEP.Citation[[43]] Supported by Conhaim's in vitro data,Citation[[6]] and Schultz's data reporting attenuated bacterial translocation and decreased base deficit in DCLHb treated septic rats,Citation[[44]] we surmised that DCLHb's small molecular size might improve distal perfusion, and result in increased O 2 uptake and decreased lactic acidosis. Similar to Crowley's data in septic dogs,Citation[[45]] a brief attenuation of systemic hypotension was seen in our study. However, in our study, DO 2, VO 2, and lactic acid were similar in the two treatment groups despite a reduction in DA dosing in DCLHb pigs (even after reaching a state of VO 2 “flow dependency”).
Study Weaknesses
First, although the cumulative dose of 700 mg/kg administered in Reah's human trialCitation[[38]] (that demonstrated a vasopressor effect in critically ill patients) was similar in scope to the 500–600 mg/kg infused in our study, this dose may have been insufficient and a continued infusion may have had a more beneficial effect. However, we did administer a higher dose of HBOC than in most other endotoxemia/SEP reports: Aranow (150 mg/kg), Bone (200 mg/kg), and Mourelatos (250 mg/kg).Citation[[33]], Citation[[34]], Citation[[36]]
Second, because this was a pilot study, it was insufficiently powered. However, the fact that essentially equivalent systemic and pulmonary vasopressor changes and adrenergic-agent sparing was found in Reah's human studyCitation[[38]] suggests that our data are accurate. These preliminary data should assist in designing a larger definitive trial that may include use of larger doses of DCLHb and addition of pulmonary vasodilators. Third, the relevance of “dopamine-resistance” in the absence of vigorous fluid resuscitation could be questioned. However, in this study, pulmonary artery catheter measurements were available prospectively; these demonstrated PCWPs of about 15–20 mm Hg. Pigs with PCWP values at the higher end may not have warranted fluid resuscitation.
Summary
A clinically relevant end stage dopamine-resistant endotoxic shock swine model is described. In comparison to salvage with hetastarch, DCLHb significantly improved MAP and allowed a reduction in dopamine dosing. However, DCLHb exacerbated pulmonary hypertension, and did not improve O 2 utilization or survival. These findings are similar to those found by other investigators of HBOCs and nonspecific NOS inhibitors in sepsis. It appears that these agents may be effective temporizing vasopressors. Despite the recently reported negative results in trauma patients,Citation[[46]] our data suggests that further evaluation of DCLHb in vasopressor-resistant sepsis is indicated and may demonstrate clinical utility. Further study might include addition of concomitant pulmonary vasodilators.
REFERENCES
- Parker M. M., Shelhamer J. H., Natanson C., Alling D. W., Parrillo J. E. Serial cardiovascular variables in survivors and non-survivors of human septic shock. Crit. Care Med. 1987; 15: 923–929
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993; 329: 2002–2012
- Schultz S. C., Grady B., Cole F., Hamilton I., Burhop K., Malcolm D. S. A role for endothelin and nitric oxide in the pressor response to Diaspirin Cross-linked Hemoglobin. J. Lab. Clin. Med. 1993; 122: 301–308
- Kilbourn R. G., Joly G., Cashon B., DeAngelo J., Bonaventura J. Cell-free hemoglobin reverses the endotoxin-mediated hyporesponsivity of rat aortic rings to alpha-adrenergic agents. Biochem. Biophys. Res. Commun. 1994; 199: 155–162
- Sielenkamper A., Martin C. M., White M., d'Almeida M., Sibbald W. J., Chin-Yee I. Efficacy of Diaspirin Cross-linked Hemoglobin, fresh blood and old blood transfusion in O 2 supply-dependent septic rats. Artif. Cells Blood Substitutes, Immobilization Biotechnol. 1996; 24: 426
- Conhaim R. L., Rodenkirch L. A., Harms B. A. DCLHb flows through compressed lung capillaries that exclude red cells. Artif. Cells Blood Substitutes, Immobilization Biotechnol. 1996; 24: 323
- Jesmok G., Gundel R. Thromoxane-blocked swine as an experimental model of severe intravascular inflammation and septic shock. Shock 1995; 4: 379–383
- Herbertson M. J., Werner H. A., Studer W., Russell J. A., Walley K. R. Decreased left ventricular contractility during porcine endotoxemia is not prevented by ibuprofen. Crit. Care Med. 1996; 24: 815–819
- Ogura H., Offner P. J., Saitoh D., Jordan B. S., Johnson A. A., Pruitt B. A., Jr., Cioffi W. G., Jr. The pulmonary effect of nitric oxide synthase inhibition following endotoxemia in a swine model. Arch. Surg. 1994; 129: 1233–1239
- Ogura H., Cioffi W. G., Offner P. J., Jordan B. S., Johnson A. A., Pruitt B. A., Jr. Effect of inhaled nitric oxide on pulmonary function after SEP in a swine model. Surgery 1994; 116: 313–321
- Weitzberg E., Rudehill A., Modin A., Lundberg J. M. Effect of combined nitric oxide inhalation and N G-nitro-L-arginine infusion in porcine endotoxin shock. Crit. Care Med. 1995; 23: 909–918
- Nava E., Palmer R.M. J., Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial?. Lancet 1991; 338: 1555–1557
- Petros A., Bennett D., Valance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet 1991; 338: 1557–1558
- Herbertson M. J., Werner H. A., Walley K. R. Nitric oxide synthase inhibition partially prevents decreased LV contractility during endotoxemia. Am. J. Phys. 1996; 270: H1979–H1984
- Roth R. I., Kaca W., Levin J. Hemoglobin: a newly recognized binding protein for bacterial endotoxins (LPS). Prog. Clin. Biol. Res. 1994; 388: 161–172
- Su D., Roth R. I., Yoshida M., Levin J. Hemoglobin increases mortality from bacterial endotoxin. Inf. Imm. 1997; 65: 1258–1266
- Vinten-Johansen J., Sato H., Zhao Z. Q. The role of nitric oxide and NO-donor agents in myocardial protection from surgical ischemic-reperfusion injury. Int. J. Cardiol. 1995; 50: 273–281
- Yang B. C., Chen L. Y., Saldeen T. G., Mehta J. L. Reperfusion injury in the endotoxin-treated rat heart: reevaluation of the role of nitric oxide. Br. J. Pharmacol. 1997; 120: 301–311
- Brandtzaeg P., Osnes L., Ovstebo R., Joo G. B., Westvik A. B., Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of Interleukin-10 as a major functional deactivator of human monocytes. J. Exp. Med. 1996; 184: 51–60
- Gardlund B., Sjolin J., Nilsson A., Roll M., Wickerts C. J., Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J. Infect. Dis. 1995; 172: 296–301
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 88: 373–376
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 27: 524–526
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1987; 84: 9265–9269
- Vallance P., Palmer R. M., Moncada S. The role of induction of nitric oxide synthesis in the altered responses of jugular veins from endotoxemic rabbits. Br. J. Pharmacol. 1992; 106: 459–463
- Jia L., Bonaventura C., Bonaventura J., Stamler J. S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 1996; 380: 221–226
- Geroulanos S., Schilling J., Cakmakci M., Jung H. H., Largiader F. Inhibition of nitric oxide synthesis in septic shock. Lancet 1992; 339: 435
- Lorente J. A., Landin L., De Pablo R., Renes E., Liste D. L-arginine pathway in the SEP syndrome. Crit. Care Med. 1993; 21: 1287–1295
- Robertson F. M., Offner P. J., Ciceri D. P., Becker W. K., Pruitt B. A., Jr. Detrimental hemodynamic effects of nitric oxide synthase inhibition in septic shock. Arch. Surg. 1994; 129: 149–155
- Booke M., Hinder F., Traber L. D., McGuire R., Traber D. L. S-ethylisothiourea, a nonamino acid inhibitor of nitric oxide synthase, reverses septic vasodilation in sheep. Shock 1995; 4: 274–281
- Hinder F., Booke M., Traber L. D., Matsumoto N., Nishida K., Rogers S., Traber D. L. Nitric oxide synthase inhibition during experimental sepsis improves renal excretory function in the presence of chronically increased atrial natriuretic peptide. Crit. Care Med. 1996; 24: 131–136
- Strand O. A., Leone A. M., Giercksky K. E., Skovlund E., Kirkeboen K. A. N G-monomethyl-L-arginine improves survival in a pig model of abdominal sepsis. Crit. Care Med. 1998; 26: 1490–1499
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc. Res. 1992; 26: 48–57
- Aranow J. S., Wang H., Zhuang J., Fink M. P. Effect of human hemoglobin on systemic and regional hemodynamics in a porcine model of endotoxemic shock. Crit. Care Med. 1996; 24: 807–814
- Bone H. G., Schenarts P. J., Booke M., McGuire R., Harper D., Traber L. D., Traber D. L. Oxalated pyridoxalated hemoglobin polyoxyethylene conjugate normalizes the hyperdynamic circulation in septic sheep. Crit. Care Med. 1997; 25: 1010–1018
- Thompson A., McGarry A. E., Valeri C. R., Lieberthal W. Stroma-free hemoglobin increases blood pressure and GFR in the hypotensive rat: role of nitric oxide. J. Appl. Physiol. 1994; 77: 2348–2354
- Mourelatos M. G., Enzer N., Ferguson J. L., Rypins E. B., Burhop K. E., Law W. R. The effects of Diaspirin Cross-linked Hemoglobin in sepsis. Shock 1996; 5: 141–148
- Krishnamurti C., Carter A. J., Maglasang P., Hess J. R., Cutting M. A., Alving B. M. Cardiovascular toxicity of human cross-linked hemoglobin in a rabbit endotoxemia model. Crit. Care Med. 1997; 25: 1874–1880
- Reah G., Bodenham A., Mallick A., Daily E. K., Przybelski R. J. Initial evaluation of Diaspirin Cross-linked Hemoglobin (DCLHb) as a vasopressor in critically ill patients. Crit. Care Med. 1997; 25: 1480–1488
- Rioux F., Petitclerc E., Audet R., Drapeau G., Fielding R. M., Marceau F. Recombinant human hemoglobin inhibits both constitutive and cytokine-induced nitric oxide-mediated relaxation of rabbit isolated aortic rings. J. Cardiovasc. Pharmacol. 1994; 24: 229–237
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann. Surg. 1991; 214: 621–626
- Shoemaker W. C., Appel P. L., Kram H. B. Oxygen transport measurements to evaluate tissue perfusion and titrate therapy: dobutamine and dopamine effects. Crit. Care Med. 1991; 19: 672–688
- Yu M., Levy M. M., Smith P., Takiguchi S. A., Miyasaki A., Myers S. A. Effect of maximizing oxygen delivery on morbidity and mortality rates in critically ill patients: a prospective, randomized, controlled study. Crit. Care Med. 1993; 21: 830–838
- Powell R. J., Machiedo G. W., Rush B. F., Jr. Decreased red blood cell deformability and impaired oxygen utilization during human sepsis. Am. Surg. 1993; 59: 65–68
- Schultz S. C., Powell C. C., Bernard E., Malcolm D. S. Diaspirin Cross-linked Hemoglobin (DCLHb) attnuates bacterial translocation in rats. Artif. Cells Blood Substitutes, Immobilization Biotechnol. 1995; 23: 647–664
- Crowley J. P., Metzger J., Gray A., Pivacek L. E., Cassidy G., Valeri C. R. Infusion of stroma-free cross-linked hemoglobin during acute gram-negative bacteremia. Circ. Shock 1993; 41: 144–149
- Sloan E. P., Koenigsberg M., Gens D., Cipolle M., Runge J., Mallory M. N., Rodman G., Jr. Diaspirin Cross-linked Hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock—a randomized controlled efficacy trial. J. Am. Med. Assoc. 1999; 282: 1857–1864