Abstract
This study is to investigate the viability of hepatocytes when transplanted into Wistar rats using co-encapsulated hepatocytes and bone marrow stem cells. Hepatocytes and bone marrow stem cells, isolated from Wistar rats, are co-encapsulated using either the standard single-step method or a novel two-step cell encapsulation method (www.artcell.mcgill.ca). After intraperitoneal transplantation into Wistar rats, the histology, fate of recovered microcapsules and viability of encapsulated hepatocytes are studied. When prepared using the standard method, there is excellent viability but only for up to 3 weeks. After this, there is extensive fibrous coating and severe fibrous adhesion and no microcapsules can be recovered. On the other hand, using the new two-step encapsulation method, the viability of the encapsulated hepatocytes can be followed for more than 4 months after transplantation. Even up to 4 months, there is significantly less host reaction when using the two-step encapsulation method and 50% of the microcapsules can be recovered. Co-encapsulated with bone marrow stem cells resulted in further increase in viability of the hepatocytes when followed up to 4 months after transplantation This new approach may improve the potential feasibility of using co-encapsulation of hepatocytes and bone marrow stem cells in bio-artificial liver support for the treatment of liver failure, especially for acute liver failure.
INTRODUCTION
Artificial cellCitation[[1]],Citation[[2]] has been used for the encapsulation of cellsCitation[[2]],Citation[[3]] to prevent host rejection after transplantation. Implantation of encapsulated hepatocytes is effective in increasing the survival time of fulminant hepatic failure.Citation[[4]] Furthermore, encapsulated rat hepatocytes could be protected from host immunorejection when implanted into mice and continue to function in the microcapsules.Citation[[5]] Studies showed that hepatocytes co-cultured with other types of cells can improve the growth and maintenance of hepatocytes.Citation[6-8] We found that the co-encapsulation of hepatocytes with bone marrow cells that contains stem cells resulted in higher and longer term viability of hepatocytes in vitro.Citation[[9]]
The present study is to investigate the transplantation into rats of co-encapsulated hepatocytes and bone marrow cells. We also compare the use of the single-step encapsulation method with the new two-step encapsulation method.
MATERIALS AND METHODS
Hepatocyte Isolation
Both hepatocytes and bone marrow cells are isolated from male Wistar rats, 250–300 g.
Bone Marrow Cell Isolation
Each rat was anaesthetized with sodium pentobarbital, and both femurs were isolated.
Iscove's Modified Dulbecco's Medium (IMDM, GIBCOBRL, Life Technologies, N.Y.) was used to flush out bone marrow cells from the femurs using a 5 ml syringe with a 22 gauge needle. The cell suspension was filtered through a nylon sieve (85 μm). The bone marrow cells were then washed with IMDM and centrifuged at 50 G for 10 min at 4 °C, this was repeated three times. After the last wash, the bone marrow cells (nucleated cells) were kept on ice until use.
Hepatocytes Isolation
Hepatocytes were isolated using the method described by Seglen.Citation[[10]] After being perfused with collagenase buffer, the liver was excised and minced in cold William's E medium (GIBCOBRL, Life Technologies, N.Y.) and shaken to free the hepatocytes. The hepatocytes were collected and filtered through a nylon sieve (85 μm), and then washed with William's E medium and centrifuged at 50 G for 5 min at 4 °C. This was repeated three times; after the last wash, the hepatocytes were kept on ice until use. The viability of cells was measured using the trypan blue exclusion test.
Encapsulation
Single-Step Encapsulation
The single-step encapsulation procedures were as described earlierCitation[[11]] but using NISCO Encapsulator, a more refined and reproducible equipment.
Hepatocytes Single-Step Encapsulation
A quantity of 10×106 hepatocytes in William's E medium were washed with buffered saline (0.85% NaCl, 10 mM HEPES, 20 mM D-fructose, PH 7.4), and suspended in 15 ml buffered saline. This cell suspension was mixed with 15 ml of 4%(w/v) stock sodium alginate (Kelco alginates LV, San Diego, CA), in a 50 ml syringe. Gel beads were made using NISCO Encapsulator (NISCO Engineering, Switzerland), adjusted to have the following parameters: 200 um nozzle; 92% amplitude; 0.25 frequency; 100% duration and 5 ml/min flow rate. The procedures after beads formation were the same as described earlier.Citation[[11]] The MW of poly-l-lysine used was 28 500 daltons (Sigma Chemical, St. Louis, MO).
Co-encapsulation of Hepatocytes with Bone Marrow Stem Cells Using Single-Setup Encapsulation
Ten ml of 10×106/ml hepatocytes were mixed with 5 ml of 10×106/ml bone marrow cells, this 15 ml of mixed cell suspension was added to 15 ml of 4% stock sodium alginate. The NISCO Encapsulator described above was used to make beads, followed by the rest of the procedures for conversion to microcapsules as described earlier.Citation[[11]]
Two-Step Encapsulation
The two-step encapsulation method follows Wong and Chang's proceduresCitation[[12]] with slight modifications. Briefly, after gelation in the 100 mM CaCl2 as in the single-step encapsulation, the alginate gel beads containing hepatocytes alone or with bone marrow cells were washed three times with buffered saline, then 15 ml of washed beads were mixed with 30 ml of 2% sodium alginate contained in a 50 ml syringe. The syringe, second droplet generator and NISCO Encapsulator were connected. The air flow rate and cell suspension extrusion rate through the NISCO Encapsulator were 4 L/ml and 3 ml/min, respectively. The beads formed were allowed to fall into a PYREX dish containing a strainer cup and 500 ml of 100 mM CaCl2. After the beads were allowed to cure in the calcium solution for approximately 5 min, the beads were immersed in 50 mg% poly-l-lysine solution for 15 min, then washed with buffered saline, and immersed into 0.2% sodium alginate for 10 min. Finally the beads were placed in 50 mM sodium citrate for 20–30 min, to solubilize the alginate gel beads inside and to form an encapsulating membrane.
Transplantation
The following four groups of Wistar male rats, 200–250 g, were transplanted intraperitoneally with a specific type of microcapsules for each group:
Group I: Single-step encapsulation hepatocytes microcapsules.
Group II: Single-step coencapsulation of hepatocytes and bone marrow cells microcapsules.
Group III: Two-step encapsulation hepatocytes microcapsules.
Group IV: Two-step coencapsulation hepatocytes and bone marrow cells microcapsules.
The above microcapsules were suspended in 10 ml 0.9% physiological saline and were either injected into the peritoneal cavity using 12 gauge needles or by means of an abdominal incision. Approximately 2×107 encapsulated hepatocytes were transplanted into each rat in the four groups, and 1×107 co-encapsulated bone marrow cells were transplanted into Groups II and IV.
Transplanted Microcapsules Recover and Hepatocyte Viability Measurement
Transplanted microcapsules were recovered by laparotomy every week for the first 8 weeks post-transplantation; thereafter, microcapsules were recovered every 2 weeks, to 16 weeks post-transplantation. The recovered microcapsules were observed microscopically and then broken mechanically to release the hepatocytes for viability testing using the Trypan blue exclusion test. In some cases, the encapsulated cells were aggregated and attached together, measurement of the hepatocytes viability was difficult, then the trypsin–EDTA (Sigma Chemical, St. Louis, MO) was applied to the cell aggregates, put in 37 °C water bath for 2–4 min until the cells detachment was observed, then added William's E Medium containing fetal calf serum to inhibit further tryptic activity, centrifuged and measured the hepatocytes viability. The average hepatocyte number in a single microcapsules in Group IV prior to and post-transplantation was determined by counting the cells in a given number of microcapsules.
Student's t-test analysis was performed on the hepatocyte viability data.
RESULTS
Microcapsule Features Prior to Transplantation
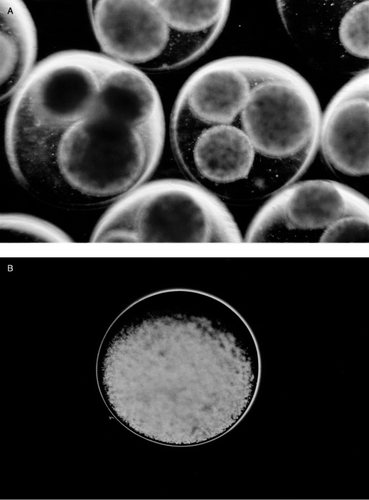
In the single-step encapsulation microcapsules, the diameter range was 0.3–0.5 mm. Under phase contrast microscope, some cells could be seen entrapped in the capsular membrane and the membrane appeared not uniform (). Microcapsules prepared using the new two-step encapsulation method, have the following characteristics. After the first step, small beads containing hepatocytes were entrapped within larger beads of 1.4–1.7 mm diameter (a). 80% of large beads each contained 3 of the smaller beads (a), with the rest containing 1–5 beads. The second step resulted in the formation of capsular membrane and the solubilization of the gels resulting in microcapsules with hepatocytes dispersed inside (b). The most important difference between these and those prepared by the single step method is as follows. Under phase contrast microscope, no cells were seen entrapped in the capsular membrane nor were there any cells exposed on the surface of the microcapsules. This translates into microcapsules with no defects in the membrane and also with no hepatocytes exposed on the surface of the microcapsules.
Figure 1. Hepatocyte microcapsules made by single-step encapsulation method; some cells could be seen entrapped in the capsular membrane (arrows).
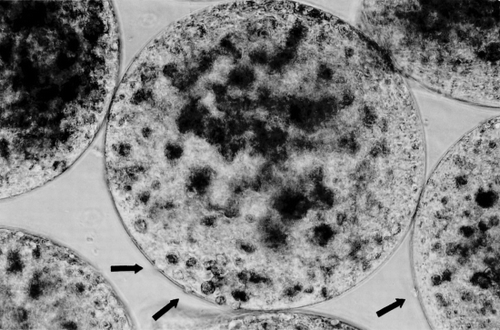
Figure 2. (a) Gel beads formed in the 100 mM CaCl2 in the first step of the two-step encapsulation method. Each large bead usually contains 3 small beads. (b) Microcapsules made by two-step encapsulation, after the inner small beads solubilize, the capsular membrane is uniform, no cells are seen entrapped in the membrane.
Morphology of Microcapsules After Implantation
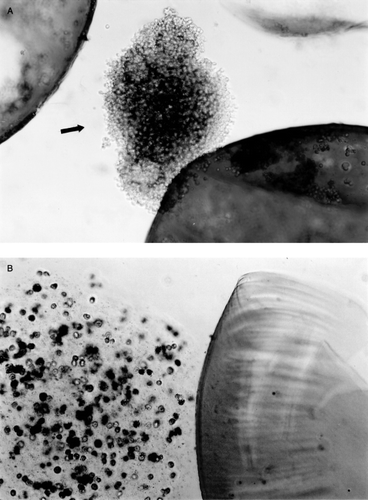
Implanted microcapsules were recovered by laparotomy. In Group I and Group II where microcapsules were prepared using the single-step method, from week 2, most of the microcapsules were seen attached to the greater omentum, the portal tract, pancreas and surface of the liver and the spleen. At week 3, nearly no intact free microcapsules could be recovered, at week 4, the aggregated microcapsules were enclosed by more connective tissue, some newly formed blood vessels were observed among the aggregates of microcapsules (a), under microscope, macrophages and lymphocytes were found infiltrating the capsular membrane (b). No further morphological and viability studies were carried out on Group I and Group II after week 4 since little or no intact microcapsules could be recovered. In contrast to the above results, when using microcapsules prepared by the new two-step method, the following results were obtained. In Groups III and IV, at week 4, more than 90% of transplanted intact microcapsules could be recovered. At 8 weeks, 12 weeks and 16 weeks, the recover rate was 60%, 50%, 20% respectively. From week 4 on some microcapsules were found attached to the greater omentum. Microscopic studies showed little or no inflammatory cell attachment or infiltration in the recovered microcapsules membrane (). Hepatocytes within the recovered microcapsules were attached to one another (a). For viability test using trypan blue, trypsin–EDTA was used to disperse the cell aggregates (b).
Figure 3. (a) Recovered microcapsules at week 4 in Group I, these hepatocytes microcapsules were made by single-step encapsulation method. The microcapsules were attached to the greater omentum and connective tissue. (b) The recovered microcapsules in a were mechanically destroyed and stained by trypan blue. The capsular membrane was infiltrated with lymphocytes and macrophages.
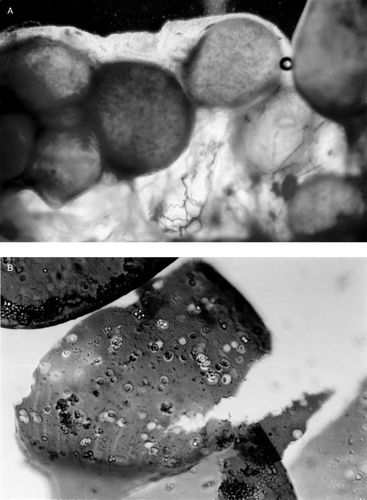
Figure 4. Recovered microcapsules made by two-step encapsulation method, at week 6 in Group IV (hepatocytes and bone marrow cells co-encapsulation). These capsular membranes were smooth; no inflammatory cells or macrophage infiltrated the membrane.
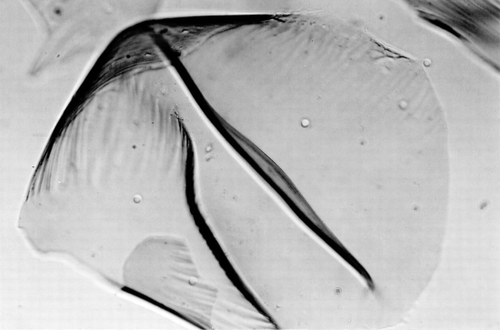
Figure 5. (a) Group IV (hepatocytes and bone marrow cells co-encapsulation by two-step method). At week 6, the recovered microcapsules were disrupted mechanically to release the content. The hepatocytes released were in an aggregated form. (b) After using Trypsin–EDTA, the aggregated cells in recovered microcapsules were dispersed and the hepatocyte viability could be determined.
Hepatocyte Viability
The average total yield of hepatocytes from one rat liver was 3.5×108. After isolation from the rats, hepatocytes viability was 88–92%, bone marrow cell (nucleated cell) viability was 98%. After the single-step encapsulation, the hepatocyte viability was 80%–85%, after the two-step encapsulation, the hepatocyte viability was 74%–78%.
Single-Step Encapsulation Method
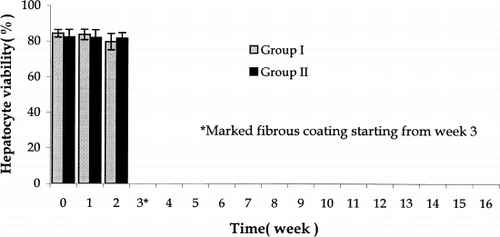
Microcapsules so prepared were tested in Groups I and II. Here, in the first two weeks post-transplantation, no differences in viability was found in the recovered hepatocytes (). However, by week 3, the foreign body reaction in the form of fibroblast and macrophage infiltration was so severe that no viability studies could be carried out after this.
Figure 6. Hepatocytes viability in recovered microcapsules in Group I and Group II. Group I: Single-step encapsulation hepatocytes microcapsules. Group II: Single-step encapsulation hepatocytes and bone marrow cells co-microcapsules. There was no significant difference of recovered hepatocytes viability up to week 2 post-implantation. *From week 3, microcapsules were infiltrated with fibrous tissue and the viability of hepatocytes could not be determined. The values are mean±SD in three rats.
Two-Step Encapsulation Method
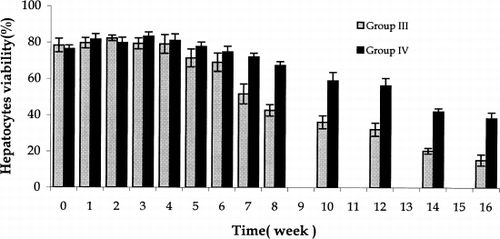
In contrast, using the new two step encapsulation method to prepare microcapsules tested in Groups III and IV the results were as followed. From week 1 to week 6 post-transplantation, there was no significant difference in hepatocytes viability (). However from week 7 to week 16 post-transplantation, the viability of hepatocytes co-encapsulated with bone marrow stem cells, Group IV, was significantly higher than in Group III where only hepatocytes were encapsulated (). In Group IV, the average hepatocytes number in a single microcapsules remained the same during the whole post-transplantation study period.
Figure 7. Hepatocytes viability in recovered microcapsules in Group III and Group IV. Group III: Two-step encapsulation hepatocytes microcapsules. Group IV: two-step encapsulation hepatocytes and bone marrow cells co-microcapsules. In Groups III and IV, from week 7 to week 16, the hepatocytes viability in Group IV was significantly higher than in Group III. The values are means±SD in three rats.
DISCUSSION
Since the 1960s, artificial cells in the form of semipermeable microcapsules have been used to encapsulate biologically active materials,Citation[[1]],Citation[[2]] and a drop method has also been developed for the encapsulation of intact cells with the aim of enclosing hepatocytes, islets and other cells.Citation[[2]],Citation[[3]] The implantation of encapsulated hepatocytes showed feasibility for the treatment of hepatic failure.Citation[[4]],Citation[[5]] However, in order for this to be practical, the encapsulated hepatocytes must remain viable for a sufficient length of time.
We found that in culture, co-encapsulation of hepatocytes with bone marrow containing stem cells improved the hepatocytes viability.Citation[[9]] In the present in vivo study, we transplanted the co-encapsulated hepatocytes and bone marrow cells intraperitoneally, and found that the co-encapsulation could also improve hepatocytes viability in vivo. From the 7th week post transplantation, the co-encapsulated hepatocytes viability was significantly higher than that of hepatoctyes encapsulation alone. We also compared the use of the single step cell encapsulation method and the new two-step encapsulation method. In the single step encapsulation method, some of the cells were often found entrapped in the capsular membrane with some exposed to the surface. This results in membrane defects and exposure of cells to the surface. The result is that after implantation the membrane defects result in fragile microcapsules. On the other hand, using the two-step encapsulation method, we were able to avoid these problems. Moreover, when these microcapsules with cells exposed on the membrane surface were implanted, there is potential for host immunorejection, resulting in infiltration by leukocytes and macrophages. However, this may not be as obvious in the present study using Wistar rat hepatocytes implanted into Wistar rats. In earlier study from this laboratory, encapsulated rat hepatocytes using the novel two step method was much better in preventing immunorejection when injected into mice.Citation[[9]] By combining the new two-step encapsulation method with the new approach of co-encapsulation of hepatocytes and bone marrow stem cells, we were able to extend the viability of the implanted hepatocytes to more that 3 months. This length of viability is compatible with supporting liver function in acute liver failure to allow for the regeneration of the liver. We are now much closer to the possible study of this approach for the support of acute liver failure.
The relation between hepatocytes and bone marrow cells has been widely studied in recent years.Citation[13-15] In our present study, the mechanism of this co-encapsulation in prolonging the viability of the hepatocytes is not clear. Since the bone marrow nucleated cells consist of different kind of cells, including stromal cells and hemopoietic stem cells, these may contribute to the improved duration of viability. In addition, further studies should be carried out on the non-syngeneic transplantation of these co-microcapsules.
In conclusion, the co-encapsulation of hepatocytes and bone marrow cells could improve hepatocytes viability when implanted into normal rats. This, together with a new two step encapsulation method has improved the useful viability of implanted hepatocytes to 4 months and therefore much closer to possible use for the support of acute liver failure.Citation[[16]]
ACKNOWLEDGMENTS
TMSC acknowledges the grant support of the Canadian Institutes of Health Research for this research project.
REFERENCES
- Chang T.M. S. Semipermeable aqueous microcapsules. Science 1964; 46: 524–525
- Chang T.M. S. Artificial Cells. Charles C. Thomas Publisher, Springfield, Ill 1972, (Also available free online www.artcell.mcgill.ca/1972covercr.htm)
- Chang T.M. S., MacIntosh F. C., Mason S. G. Semipermeable aqueous microcapsules: preparation and properties. Can. J. Physiol. Pharmacol. 1966; 44: 115–128
- Wong H., Chang T.M. S. Bioartificial liver: implanted artificial cells microcapsulated living hepatocytes increases survival of liver failure rats. Int. J. Artif. Organs 1986; 9: 335–336
- Wong H., Chang T.M. S. The viability and regeneration of artificial cell microencapsulated rat hepatocyte xenograft transplants in mice. J. Biomater., Artif. Cells, Artif. Organs 1988; 16: 731–740
- Langenbach R., Malick L., Tompa A., Charles Kuszynski C., Freed H., Huberman E. Maintenance of adult rat hepatocytes on C3H/10T1/2 Cells. Cancer Res. 1979; 39: 3509–3514
- Michalopoulos G., Russell F., Biles S. Primary cultures of hepatocytes on human fibroblasts. In Vitro 1979; 15: 796–806
- Guguen-Guillouzo C., Clement B., Baffet G., Beaumont C., Morel-Chany E., Glaise D., et al. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp. Cell Res. 1983; 143: 47–54
- Liu Z. C., Chang T.M. S. Effects of bone marrow cells on hepatocytes: when co-cultured or co-encapsulated together. Artif. Cells, Blood Substitutes Immobilization Biotechnol. 2000; 28(4)365–374
- Seglen P. Q. Preparation of isolated rat liver cells. Methods Cell Biol. 1976; 13: 23–83
- Wong H., Chang T.M. S. The microencatsulation of cells within alginate poly-l-lysine microcapsules prepared with the standard single step drop technique: histologically identified membrane imperfections and the associated graft rejection. Biomater., Artif. Cells, Immobilization Biotechnol. 1991; 19(4)675–686
- Wong H., Chang T.M. S. A novel two step procedure for immobilizing living cells in microcapsules for improving xenograft survival. Biomater. Artif. Cells, Immobilization Biotechnol. 1991; 19(4)687–697
- Theise N. D., Nimmakayalu M., Gardner R., Illei P. B., Morgan G., Teperman L., et al. Liver from bone marrow in humans. Hepatology 2000; 32: 11–16
- Theise N. D., Badve S., Sazena R., Henegariu O., Sell S., Crawford J. M., et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2001; 31: 235–240
- Alison M. R., Poulsom R., Jeffery R., Dhillon A. P., Quaglia A., Jacob J., et al. Hepatocytes from non-hepatic adult stem cells. Nature 2000; 406: 257
- Chang T.M. S. Editorial: bioencapsulated hepatocytes for experimental liver support. J. Hepatol. 2001; 34: 148–149