Abstract
A theoretical model is developed to investigate the influence of hemoglobin-based oxygen carriers (HBOCs) on oxygen transport in capillary-size vessels. A discrete cell model is presented with red blood cells (RBCs) represented in their realistic parachute shape flowing in a single file through a capillary. The model includes the free and Hb-facilitated transport of O2 and Hb–O2 kinetics in the RBC and plasma, diffusion of free O2 in the suspending phase, capillary wall, interstitium and tissue. A constant tissue consumption rate is specified that drives the simultaneous release of O2 from RBC and plasma as the cells traverse the capillary. The model mainly focuses on low capillary hematocrits and studies the effect of free hemoglobin affinity, cooperativity and concentration. The results are expressed in the form of cell and capillary mass transfer coefficients, or inverse transport resistances, that relate the spatially averaged flux of O2 coming out of the RBC and capillary to a driving force for O2 diffusion. The results show that HBOCs at a concentration of 7 g/dl reduce the intracapillary transport resistance by as much as 60% when capillary hematocrit is 0.2. HBOCs with high O2 affinity unload most O2 at the venular end, while those with low affinity supply O2 at the arteriolar end. A higher cooperativity did not favor O2 delivery due to the large variation in the mass transfer coefficient values during O2 unloading. The mass transfer coefficients obtained will be used in simulations of O2 transport in complex capillary networks.
INTRODUCTION
Hemoglobin (Hb) is the protein in red blood cells (RBCs) responsible for the transport of oxygen. Hemoglobin molecules extracted from RBCs cannot be used without modification as oxygen carriers as the protein molecules break down in the body and are toxic to the kidneys. Over the last decade, advances in biotechnology such as cross-linking, recombinant modification, and encapsulation have overcome this problem and have resulted in the development of hemoglobin-based oxygen carriers (HBOCs) that can temporarily replace whole blood transfusions. Experiments with HBOCs in the plasma have shown an overall improvement in the delivery of O2 to the tissue, Citation[[1]] particularly in cases where the fraction of RBCs (hematocrit) in the blood is small, in sickle cell anemia, for perioperative uses in surgery, resuscitation after traumatic blood loss, and increased oxygen delivery to ischemic tissues. The main advantages of HBOCs are: 1) the blood substitutes can be sterilized of microorganisms such as those causing AIDS and hepatitis; 2) substitutes do not need to be cross-matched or typed to blood group antigens, thus allowing on-spot transfusions; 3) HBOCs can be lyophilized and stored as stable dry powder for a long time and reconstituted with salt solution just before use; and 4) HBOCs can be made using non-human hemoglobins, e.g. bovine or recombinant Hb. At present, a number of clinical trials are ongoing, but there is no FDA approved blood substitute for use in humans.
Until recently, intraluminal resistance to oxygen transport was neglected in modeling studies under the tacit assumption that the resistance to mass transfer within the tissue was the dominant factor.Citation[[2]] Initial studies that did consider the intraluminal resistance treated blood as a continuum. Later studies using discrete cell models showed that radial convection currents were not important, and that in small capillaries transport of oxygen occurs primarily in the radial direction through the narrow plasma layer between the RBC and the capillary wall via passive diffusion. The discrete cell model was further advanced by studies on the effect of cell spacing related to hematocritCitation[[3]] and the shape of the RBC.Citation[[4]] A more detailed model that included muscle tissue was presented by Groebe (1995)Citation[[5]] that comprised of three concentric regions. The innermost region represented the RBC, the second, the carrier free region, and the outermost, the muscle region. The carrier free region was a lumped region comprising the plasma layer, the capillary wall, and the interstitial space just outside the wall. Comprehensive reviews are available on the development of mathematical models that predict the distribution of O2 in tissues.Citation[[2]], Citation[[6]] An accurate computational model of oxygen transport in the presence of HBOCs should help in interpreting results of animal experiments and clinical trials and provide a fundamental understanding of the transport processes in the microvasculature. Past efforts in this area have been focused on arteriolar-sized vessels under in vitro conditions.Citation[[7]] In the microvasculature of the hamster retractor muscle, it has been observed that two-thirds of oxygen is exchanged in the arterioles and the rest in capillaries.Citation[[8]] Measurements in other tissues such as the brain show that cortical capillaries unload about twice the amount of oxygen to the brain tissue as compared to arterioles.Citation[[9]] In the heart under physiological conditions precapillary oxygen losses appear to be small.Citation[[10]] Since the contribution of oxygen from capillaries is found to be fairly substantial in any given tissue, the present paper focuses on this topic by describing the fundamental transport processes associated with RBC/HBOC mixtures in capillaries. We perform simulations on the hamster retractor muscle since most biophysical parameters are available for this tissue and because of our previous calculations under physiological conditions.Citation[[11]], Citation[[12]]
An additional feature of the present model is consideration of relative differences in plasma and RBC velocities. The fluid dynamics of plasma has been extensively investigated via semi-analytical and numerical studiesCitation[[13]] and the overall conclusion has been that the effect of plasma motion on local oxygen transport is insignificant. This might be true for cases of high hematocrit where the plasma gaps are small, but for cases of low hematocrit, a frequently encountered clinical condition, the effect of convection cannot be ignored as will be demonstrated by results obtained for the tissue PO2 distribution. In this paper, we predict the transport of free and bound oxygen in capillaries by considering both diffusion and convection for the low hematocrit cases. Also, the present model uses a parachute shape for the RBC that is based on calculations using an elastic model of the membrane.Citation[[14]] The calculations take into account both the radial and axial drop in oxygen tension for the capillary segment.
This is the first model of capillary transport that includes HBOCs (see Appendix). The calculations consider the full pathway of oxygen transport from its source in the hemoglobin molecule to its sink in the mitochondria of the parenchymal cells. The model uses Krogh-type cylindrical tissue geometry; the goal of the model is calculating capillary mass transfer coefficients that will be used in simulations of O2 transport in complex capillary networks.
MATHEMATICAL MODEL
Capillary Segment Geometry
An axisymmetric model of the capillary and the surrounding tissue is used in the present study. The computational domain consisted of five sub-regions: the red blood cell, plasma, vascular wall, interstitium, and the tissue (see ). The shape of the red blood cell has been selected based on recent numerical simulationsCitation[[14]] of RBC flow in capillaries that take into account the deformation of the RBC membrane. Each RBC was represented by a cylinder with a hemispherical leading end and an inverted cap at the trailing end and with smoothed edges. For a single RBC, we define a unit cell with a length, where V rbc is the volume of the RBC, R p is the radius of the capillary, and H c is the capillary (tube) hematocrit. In microcirculatory experiments, it is common to measure lineal cell density, LD=L −1, the number of RBCs per unit length of capillary; capillary hematocrit can then be expressed in terms of LD using Eq. 1 if capillary diameter and RBC volume are known. A hematocrit range of 0.2 to 0.4 is considered in this work that corresponds to a lineal cell density between 36 and 72 cells/mm. Under normal physiological conditions the average LD=64 cells/mmCitation[[15]] corresponds to a systemic hematocrit H sys=0.51. Using Eq. 1 we estimate H c≈0.35. Recent data on hemodilution in hamster retractor muscle from H sys=0.53 to 0.15 show the decrease of LD from 56 cells/mm to 47 cells/mm.Citation[[16]] Therefore, the range of LD=36–72 cells/mm chosen in this study covers the physiological range of this muscle.
Model Assumptions
In formulating the model, we make the following major assumptions (a more complete list of assumptions and their discussion can be found in Popel [1989].Citation[[6]]
The geometry of the problem as described in the section above.
The model considers a train of three equidistant RBCs.
The plasma motion is in steady state in the coordinate system moving with the RBCs and is characterized by the velocity components, v z and v r.
In the tissue, myoglobin and oxygen are assumed to exist in local chemical equilibrium,
where S Mb is the fractional saturation of Mb, and P 50 Mb is the partial pressure of O2 corresponding to 50% saturation of myoglobin. Although the oxymyoglobin dissociation rate is 66 s −1Citation[[3]] comparable to that of oxyhemoglobin, the reaction does not lead to a chemical boundary layer region in the muscle fiber, since the flux density of oxygen is distributed over a surface area larger than in the RBC.
O2 consumption in the entire tissue is assumed to be constant. This assumption is appropriate, since P cr=0.5 Torr, e.g.,Citation[[17]] and since tissue pO2 does not fall below 3.24 Torr in all the simulations.
Navier–Stokes Equation for Plasma Flow
The equations describing the transient mass transport for free and facilitated oxygen transport in all five sub-regions of the computational domain are presented in Appendix. In order to express the convective component of the intracapillary mass transport equation, it is necessary to know the velocity profile in the plasma. The fluid dynamics of the plasma was described by the equation of continuity or mass balance and the equation of motion, which describes the momentum balance. Plasma was considered to be a Newtonian fluid with a constant density ρ and a constant viscosity μ. These assumptions lead to the Navier–Stokes equation, and for low Reynolds number (Re<0.1) the equation can be simplified to Stokes flow.
The plasma flow has been modeled to take into account the leak back from the individual cells. The amount of leak back depends on the plasma sleeve thickness and the velocity of the red blood cell, v c. The frame of reference in this study is centered on the middle of the RBC and is moving with the RBC from left to right. Hence, in this reference frame the tissue is moving right to left with a velocity of −v c. For a capillary hematocrit of 0.2, the calculated pressure drop is approximately 4×10 −3 Torr/μm for the case where no HBOC was present, which is consistent with experimentally measured average pressure drops of 6×10 −3 Torr/μm in the cat mesenteric microcirculation.Citation[[18]], Citation[[19]] Note that explicitly accounting for the endothelial glycocalyx in these calculations leads to significantly higher values of pressure drop.Citation[[14]]
Solution Method
The system of partial differential equations was solved using a finite-element method (FEM) implemented with the finite-element program FLEXPDE© (PdeSolutions, Inc., Antioch, CA). The complete domain was divided into 5 sub-regions: the RBC, plasma, wall, interstitium and the tissue. In order to eliminate possible edge effects due to boundary conditions imposed at the two ends of the domain, a train of 3 RBCs was taken as part of the computational domain.Citation[[4]] Mass transfer coefficients and other transport properties such as wall-average, RBC-average and plasma-average PO2 were calculated for the middle cell only. The O2 tension P and normal O2 flux (−Dα∂P/∂r) were taken to be continuous throughout the domain, while variables Sc Hb and SsHb were constrained to the RBC and plasma, respectively. However, to avoid internal boundaries in the computational domain for ScHb and SsHb, all species were taken to be continuous throughout the domain by setting the diffusivities of RBC and plasma hemoglobin to be very small outside their respective domains. The initial value of ScHb was set to zero everywhere except in the RBC, and thus Sc Hb≈0 for all time in the plasma, wall, interstitium and tissue regions. Similarly, SsHb≈0 everywhere except in the plasma. The equations for P, ScHb, and Ss Hb were solved in the entire cylindrical domain with the conditions of continuity for PO2 and normal flux of O2 at the cell-plasma, plasma-wall, wall-interstitium and interstitium-tissue interfaces being satisfied automatically.
The goal of this paper was to study the process of O2 unloading within a wide range of PO2 values. Thus, in most of the work presented here we start with an initial PO2 of 100 Torr and follow the RBCs and plasma until the average RBC saturation has dropped to ~0.1. The length of the “capillary” in this case is unrealistically large and is only used as a means of studying variation of mass transfer coefficients over a wide range of saturation values. Some of the results, especially those of hypoxia are presented for the average capillary length in the hamster retractor muscle. The partial differential equations were solved with an initial time step of 10−10 s that was gradually increased to ~0.2 s during the course of the total simulation time. An isoparametric formulation was used for solving the discretized FEM model of the fluid dynamics in the plasma region with ~1400 triangular elements and nodal vz, vr and hydraulic pressure, pH, being the primary degrees of freedom. For solving the mass transport equations, the isoparametric formulation contained ~9000 triangular elements with primary degrees of freedom being P, ScHb and SsHb. The conjugate-gradient method and a second-order Galerkin time step scheme were used in the calculations. The conjugate-gradient method iterates to a tolerance of 10−6. The mass balance for the tissue region was satisfied to within 0.1%. The accuracy of the program was validated by comparing numerical and analytical solutions of the axial PO2 profile calculated using Eqs. A7–A10 14-17 for the case when there was no HBOC in plasma, and for the case where the cooperativities of the HBOC and RBC oxyhemoglobin were the same. In the first case, the numerical and analytical values agreed to within 0.001% of each other and in the second, they agreed to within 0.005% of each other.
Model Parameters
In order to solve the Navier–Stokes equations for the flow of plasma, it is necessary to know the viscosity of the HBOC-plasma solution. The viscosity varies with hemoglobin concentration and can be determined using the fit of Mooney’s relationship to experimental dataCitation[[20]] where μ s=1.2 cP is the viscosity of plasma without hemoglobin,Citation[[21]] and [Hb] s is the concentration of hemoglobin in the plasma (g/ml). Constants A and B are adjustable parameters of the equation determined from an experimental fit of data, having values of 2.77 ml/g and 1.2 ml/g, respectively, for human tetrameric Hb solution.Citation[[20]]
The values of the parameters used in Eqs. A1–A6 8-13 are summarized in . A number of these parameters have been discussed in Roy and Popel [1996].Citation[[22]] The vascular wall and the tissue consumption are taken to be 10−4mlO2ml−1s −1Citation[[23]] and 1.58×10 −4 mlO2ml−1s−1,Citation[[15]] respectively. The diffusion coefficient of myoglobin was taken from Wang et al. [1997].Citation[[24]] In Eqs. A3–A6 10-13, we require the diffusion coefficients of the HBOC, DHb, and O2, Do, as functions of HBOC concentration. For the purposes of this study, we analyzed data for DHb and Do as measured by Spaan et al. [1980],Citation[[25]] Nishide et al. [1997],Citation[[26]] and Bouwer et al. [1997].Citation[[27]] There was an overall agreement in the measurements over a range of 0 to 40 g/dl hemoglobin concentration. The most recent set of diffusion coefficient valuesCitation[[27]] were used in the paper.
Table 1. List of Model Parameters
Definition of Mass Transfer Coefficients
A mass transfer coefficient is calculated as the ratio of the O2 flux through a surface to the difference in O2 tension that is the driving force for diffusion. We define two mass transfer coefficients, capillary mass transfer coefficient, k o, and a cell mass transfer coefficient, kcell. In a discrete model representation, it is possible to separately calculate the O2 flux leaving the RBC and the total flux reaching the luminal surface of the capillary. The cell mass transfer coefficient, kcell, is defined as where
is the surface-averaged flux leaving the RBC,
is the volume-averaged PO2 of the RBC, and
is the volume-averaged PO2 of the plasma.
The capillary mass transfer coefficient, ko, is defined as where
is the surface-averaged flux reaching the luminal surface of the capillary and
is the surface-averaged plasma-wall interface PO 2. The mass transfer coefficients are useful in transport studies of vascular networks where it is necessary to quantify the intracapillary transport resistance of O2 in the presence and absence of HBOCs.
The relative contribution of O2 flux from the RBC to the total flux at the wall can be represented by the parameter λ. Note that because the tissue consumption rate is constant, the total wall flux is constant by mass balance along the length of the capillary. Thus where Aw is the surface area of the capillary wall within a unit cell and
the flux contribution from only the HBOC in the plasma. Therefore
where 0≤λ≤1.
RESULTS
The oxygen releasing properties of hemoglobin depend on the affinity to O2 and its cooperativity. Hence, the parametric study involved changing the values of P50,sHb, ns, and other model parameters like HBOC concentration [Hb]s, and cell spacing that are likely to influence the PO2 distribution in the tissue.
At first, we varied the initial condition by changing the PO2 value specified on the left side of the capillary domain at t=0. The calculations were terminated when PO2 values fell below ~10 Torr for a given set of model parameters. The goal of these calculations was to consider the possible effects of different initial PO 2 values on the mass transfer coefficients. The capillary mass transfer coefficient, ko, is presented as a function of RBC saturation, ScHb, for P 50,sHb=29.3 Torr (). After the initial transients, the values of ko were found to coincide for two simulations that began with PO2 values of 40 and 100 Torr on the left side of domain. Hence, for a given set of model parameters there is a unique relationship between the capillary mass transfer coefficient and RBC saturation independent of initial conditions. The cell mass transfer coefficient, kcell, was also found to be insensitive to initial conditions. Consequently, throughout the paper we present ko and kcell as a functions of RBC oxyhemoglobin saturation, ScHb.
Figure 2. Comparison of ko, for different initial PO2. P50,cHb=P50,sHb=29.3 Torr, nc=ns=2.2, [Hb]s=7g/dl, Hc=0.2.
![Figure 2. Comparison of ko, for different initial PO2. P50,cHb=P50,sHb=29.3 Torr, nc=ns=2.2, [Hb]s=7g/dl, Hc=0.2.](/cms/asset/6b8827f4-80cd-4666-94b0-eed44020bb02/ianb19_a_11116809_uf0002_b.gif)
Effect of HBOC Oxygen Affinities on k o and k cell
In the absence 2,3-diphosphoglycerate (2,3-DPG) that cross-links the two β-chains of the hemoglobin molecule in the RBC, the value of P 50,s Hb is known to be around 8 Torr, an unfavorably high oxygen affinity that may lead to an insufficient release of oxygen in the body. Hence, in order to improve the oxygen releasing capacity of extracellular hemoglobin, it is necessary to chemically modify the hemoglobin protein.Citation[[1]] Typically, the P 50,sHb for these modified hemoglobins ranges between 27 and 38 Torr depending on the site targeted, mode of modification, and in some cases the source of protein. In this paper, we study the dynamics of oxygen unloading for P50,s Hb=10, 20, 29.3, 40 and 50 Torr while P50,cHb is held constant at 29.3 Torr. Thus, it will be possible to understand the variation in the mass transfer coefficients and tissue PO2 distribution for a wide range of P50,sHb values and RBC oxygen saturation.
The variation of the capillary mass transfer coefficient, ko, versus RBC saturation, ScHb, for different values of P50,sHb is presented in A. The value of k o varied over a larger range for P50,sHb values less than 29.3 Torr, while remaining nearly constant for values greater than 29.3 Torr. For the case where HBOC concentration is zero, the value of ko varied in the range 0.97–1.2×10−6 mlO2s−1Torr −1cm −2 and was lower than all the HBOC cases for the entire range of ScHb values. For hemoglobin solutions having a P50,s Hb=10 Torr, the capillary mass transfer coefficient, ko increased monotonically four-fold from a value of 1×10 −6mlO2s−1Torr−1cm−2, as ScHb decreased from 0.8 to 0.1. For P50,sHb=50 Torr, k o decreased from 1.7×10−6 mlO2s −1Torr −1cm−2 to 1.4×10−6 mlO2s−1Torr −1cm−2 as ScHb decreased from 0.8 to 0.1.
Figure 3. (A) Comparison of k o for different values of HBOC oxygen affinities. P 50,cHb=29.3 Torr, nc=ns=2.2. Hc=0.2, [Hb] s=7 g/dl. (B) Comparison of kcell for different values of HBOC oxygen affinities. P50,cHb=29.3 Torr, nc=ns=2.2, Hc=0.2, [Hb] s=7 g/dl.
![Figure 3. (A) Comparison of k o for different values of HBOC oxygen affinities. P 50,cHb=29.3 Torr, nc=ns=2.2. Hc=0.2, [Hb] s=7 g/dl. (B) Comparison of kcell for different values of HBOC oxygen affinities. P50,cHb=29.3 Torr, nc=ns=2.2, Hc=0.2, [Hb] s=7 g/dl.](/cms/asset/715303b8-5e9c-4344-814e-eca5a460aa1c/ianb19_a_11116809_uf0003_b.gif)
The variation of kcell is presented in B. For the case where no HBOC is present, k cell rose from 0.034×10−6mlO 2s−1Torr −1cm−2 to 0.19×10−6 mlO2s−1Torr −1cm−2 as ScHb decreased from 0.8 to 0.1. For all HBOCs, kcell increased as Sc Hb decreased, with the maximum change for P 50,sHb=10 Torr, where the variation in kcell was between 0.78 and 1.8×10 −6mlO2s−1Torr −1cm−2. The least change in kcell values was observed for P 50,sHb=50 Torr, with the values varying between 0.8 and 1.1×10−6 mlO2s−1Torr−1cm −2.
Effect of HBOC Cooperativity on ko and k cell
The effect of changing HBOC cooperativity, n s, is studied by varying the value of ns at 1.0, 2.2 and 3.5. The variation of the capillary mass transfer coefficient, ko, versus average RBC oxygen saturation value, ScHb, is presented in A. The results are compared to the case where no HBOC is present in plasma. When ns=1.0 (non-cooperative binding that is encountered in some polymerized hemoglobins), k o increased monotonically from 1.4×10 −6 mlO2s −1Torr −1cm −2 to 2.05×10 −6 mlO2s −1Torr −1cm −2 as S c Hb decreased from 0.8 to 0.1. When n s=3.5, k o initially rose from 1.35×10 −6 mlO 2s −1Torr −1cm −2 to 2.7×10 −6 mlO 2s −1Torr −1cm−2 as Sc Hb decreased from 0.8 to 0.44 and thereafter decreased to 1.7×10 −6 mlO2s−1Torr −1cm −2 as Sc Hb further decreased to 0.1. The rapid changes in ko could be attributed to the high degree of non-linearity in the Hill dissociation curve caused by the high value of n s. The corresponding values of the cell mass transfer coefficient, k cell, are presented in B. For n s=1, k cell increased from 0.79×10 −6 mlO 2s −1Torr −1cm −2 to 1.27×10 −6 mlO 2s −1Torr −1cm −2. For n s=3.5, k cell reached a peak value of 1.41×10 −6 mlO 2s −1Torr −1cm −2 at S c Hb=0.37 and then decreased to 1.15×10 −6 mlO 2s −1Torr −1cm −2 as S c Hb decreased to 0.1. Note that the peak values of k o and k cell do not occur at the same value of S c Hb.
Effect of HBOC Concentration on k o and k cell
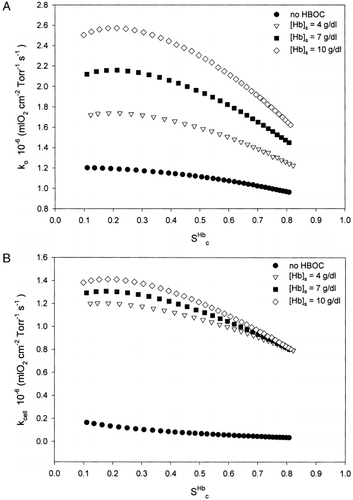
The amount of oxygen being carried by the plasma will depend on the amount of HBOC present in it and the capillary hematocrit. In order to understand the effect of concentration on the mass transfer coefficients and PO 2 distribution, we vary the HBOC concentration between 0 and 10 g/dl keeping all other parameters constant.
The results for the capillary mass transfer coefficient for different HBOC concentrations are presented in A. As the HBOC concentration increased from 0 to 10 g/dl, the value of k o was progressively higher at a corresponding value of S c Hb. For the case where no HBOC is present, k o increased by 25% from a value of 0.97×10 −6 mlO 2s −1Torr −1cm −2 as S c Hb decreased from 0.8 to 0.1, while for the case where [Hb] s=10 g/dl, k o increased by 58% from a value of 1.63×10 −6 mlO 2s −1Torr −1cm −2 for the same range of S c Hb values. The value of k cell in B followed a trend similar to that of k o.
Effect of HBOC Affinity, Cooperativity, and Concentration on Tissue Oxygen Distribution
In order to better understand the effect of HBOC properties on tissue oxygenation, the histograms of tissue PO 2 over the entire capillary length of 417 μm corresponding to the hamster retractor muscle are presented in . Note, that the calculations were carried out with a low capillary inlet PO 2 of 35 Torr, reflecting a high degree of precapillary O 2 transportCitation[[15]] and a higher muscle consumption rate of 2.76×10 −4 mlO 2ml −1s −1 for all the runs in order to understand the behavior of HBOCs under conditions of relatively low PO 2 with the concentration of HBOC [Hb] s set at 7 g/dl. The mass transfer coefficients k o and k cell were higher by 5–10% when compared to the values shown in A–B, A–B and A–B. The most notable differences between the cases are in the spread and peak values of the distribution. The effect of HBOC affinity is shown in A, where in the absence of HBOC, the tissue PO 2 ranged between 3 Torr and 30 Torr with 23% of the tissue having a PO 2 between 15 and 20 Torr with a mean PO 2 of 16 Torr. For P 50,s Hb=10 Torr, tissue PO 2 ranged between 10 and 30 Torr with 34% of tissue having PO 2 between 15 and 20 Torr, similar to the case with no HBOC but with a higher mean PO 2 of 20 Torr. For P 50,s Hb=29.3 Torr, matching that of the hemoglobin in the RBC, tissue PO 2 was distributed over an even smaller range of 20 and 35 Torr with 36% of the tissue having PO 2 between 20 and 25 Torr with a mean PO 2 of 23 Torr. The distributions shift to the right towards higher PO 2 values as the HBOC oxygen affinities decreases. When P 50,s Hb was increased even further to 50 Torr, the PO 2 spread was between 10 and 35 Torr with a mean PO 2 of 21 Torr. The increase in the spread of PO 2 values is due to the early unloading of oxygen by the HBOC having a high P 50,s Hb. Also presented in B are radial profiles at the arteriolar-end (indicated by B), the middle of the capillary segment (indicated by M), and towards the end of the capillary (indicated by E). The early unloading by the HBOC having P 50,s Hb=50 Torr is comparable to that of P 50,s Hb=29.3 Torr. Since the HBOC having P 50,s Hb=50 Torr unloads most of its oxygen early on while the HBOC having P 50,s Hb=10 Torr does not unload its oxygen until PO 2 values have fallen to very low values, the radial profiles at the venular end for both these HBOCs is much lower than for the case where P 50,s Hb=29.3 Torr.
Figure 6. (A) Tissue PO 2 distribution along capillary length for different HBOC oxygen affinities. P 50,c Hb=29.3 Torr, n c=n s=2.2, H c=0.2, [Hb] s=7 g/dl. (B) Radial tissue PO 2 distribution profiles for different HBOC oxygen affinities. P 50,c Hb=29.3 Torr, n c=n s=2.2, H c=0.2, [Hb] s=7 g/dl. The vertical line shows the plasma-wall interface. A-arteriolar end, M-midcap, V-venular end. –·–·–·–· P 50,s Hb=10 Torr, ------- P 50,s Hb=29.3 Torr, ——— P 50,s Hb=50 Torr.
![Figure 6. (A) Tissue PO 2 distribution along capillary length for different HBOC oxygen affinities. P 50,c Hb=29.3 Torr, n c=n s=2.2, H c=0.2, [Hb] s=7 g/dl. (B) Radial tissue PO 2 distribution profiles for different HBOC oxygen affinities. P 50,c Hb=29.3 Torr, n c=n s=2.2, H c=0.2, [Hb] s=7 g/dl. The vertical line shows the plasma-wall interface. A-arteriolar end, M-midcap, V-venular end. –·–·–·–· P 50,s Hb=10 Torr, ------- P 50,s Hb=29.3 Torr, ——— P 50,s Hb=50 Torr.](/cms/asset/4c781fd9-4fcc-47e5-9ee5-a27ccfd73fe3/ianb19_a_11116809_uf0006_b.gif)
Figure 7. Tissue PO 2 distribution along capillary length for different HBOC oxygen cooperatives. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=2.2, H c=0.2, [Hb] s=7 g/dl.
![Figure 7. Tissue PO 2 distribution along capillary length for different HBOC oxygen cooperatives. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=2.2, H c=0.2, [Hb] s=7 g/dl.](/cms/asset/66f39bb3-7f86-4247-bd8f-0c9378b55e35/ianb19_a_11116809_uf0007_b.gif)
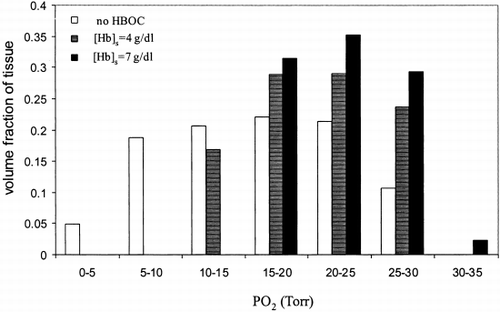
Figure 8. Tissue PO 2 distribution along capillary length for different HBOC concentrations. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=n s=2.2, H c=0.2.
Similar to A the tissue PO 2 distributions for different cooperativities with the same inlet capillary PO 2 of 35 Torr are presented in . For n s=1 the mean PO 2 is 23.5 Torr with 34% of the tissue having a PO 2 between 20 and 25 Torr. For n s=3.5, 47% of the tissue PO 2 is between 25 and 30 Torr with a mean PO 2 of 27 Torr.
The effect of HBOC concentration on tissue PO 2 distribution is presented in for HBOC concentrations of 0, 4 and 7 g/dl. Similar to A and , the calculations were carried out with the same initial PO 2 and the results are distinguishable from one another by their range and mean PO 2 values. In the presence of 4 g/dl HBOC, tissue PO 2 was spread over a range of 10 to 30 Torr with 60% between 15 and 25 Torr and mean a PO 2 of 21 Torr. The overall distribution shifted to the right as concentration increased.
Effect of Hematocrit
The results for different hematocrits for the case where [Hb] s=7 g/dl and with other parameters kept constant are presented in A and B. The value of k o in A increased at corresponding values of S c Hb as capillary hematocrit increased from 0.2 to 0.4 (lineal cell density, LD, increased from 36 to 72 cells/mm). The largest increase in k o of 60% was observed for a hematocrit of 0.4, while the smallest change of 50% was observed for a hematocrit of 0.2. The decrease in intracapillary resistance with increase in hematocrit is attributed to the shortening of the plasma gap length. This effect was also found for the case when no HBOC was present in plasma. The cell mass transfer coefficient, k cell, displayed a trend similar to k o with the greatest increase observed for a hematocrit of 0.4 and the least for a hematocrit of 0.2.
Effect of Oxygen Dissociation Rates on k o
We also studied the influence of kinetic dissociation rates of hemoglobin. In , the capillary mass transfer coefficient, k o, is presented for the cases of chemical equilibrium and nonequilibrium, in the presence and absence of HBOCs in plasma. Generally, the assumption of instantaneous chemical equilibrium within the RBC is valid as long as the rate of the chemical reaction is much faster than the diffusive transport. Assuming chemical equilibrium greatly simplifies the system of equations being solved and thus speeds up the solution of the problem. However, this assumption breaks down near the RBC membrane because oxygen is permeable to the RBC membrane while the hemoglobin molecule is not thus creating a boundary layer on the inside of the RBC membrane. In the presence of HBOC in the plasma, there are two additional boundary layers formed, one near the RBC-plasma interface and the other near the plasma-wall interface due to the inability of HBOC to diffuse into the RBC and capillary wall. The effect of the additional boundary layers on the plasma side can be seen in values of k o in . In the absence of HBOC, k o differs by as much as ~20% between equilibrium and non-equilibrium cases, whereas in the presence of HBOCs the corresponding difference is as much as ~55%. Thus, the kinetic effects may play some role in capillary transport. The effect should be more pronounced for hemoglobins with slower kinetics.
Effect of Plasma Convection and HBOC-Facilitated Diffusion
The effect of plasma convection terms in Eqs. A3 and 10 and A411and A1118 on the numerical solution was studied by estimating the mean tissue PO2 in the presence and absence of HBOC. In the absence of convection terms, the mean tissue PO 2 was lower by 0.6 Torr with HBOC (concentration of 7g/dl and hematocrit of 0.2) and by 1.1 Torr in the absence of HBOC when compared to the corresponding cases when convection terms were included in the numerical solution. Thus, the plasma convection plays only a minor role in O2 delivery to tissue.
The importance of HBOC facilitated O2 transport in the plasma was studied by varying the value of D Hb in Eq. A411 between 1×10−12 and 1×10−6 cm 2/s in the absence of convection terms in Eqs. A3 10 and A411. A comparison of the capillary mass transfer coefficient for all the cases is presented in . The capillary mass transfer coefficient was found to decrease as DHb is decreased from 1×10 −6 cm2/s to 1×10 −12 cm2/s, indicating an increase in the overall intravascular O 2 transport resistance. The resistance increased by 8% as D Hb was lowered from 7.28×10 −7 to 1×10 −12. The capillary mass transfer coefficient is nearly the same for D Hb=10 −9 and 10 −12 cm 2/s. Hemoglobin tetramers have a diffusion coefficient between 5×10 −7 and 8×10 −7 cm 2/s, and polymerized hemoglobins have a diffusion coefficient between 5×10 −8 and 2×10 −7 cm 2/s.Citation[[26]] The value of D Hb in all our simulations is 7.28×10 −7 cm 2/s, and since there is very little change in the capillary mass transport resistance for diffusivities lower than 7.28×10 −7 cm 2/s, it is unlikely that changing the diffusivity of the hemoglobin molecule will have a significant effect on the overall transport of oxygen to the tissue thus no effect of facilitated diffusion. As the total flux is constant for all the cases, we observe a corresponding increase in the driving force as D Hb decreases. The mean tissue PO 2 for a diffusion coefficient of 1×10 −6 was 0.23 Torr more than when diffusion coefficient was 1×10 −12.
DISCUSSION
The capillary mass transfer coefficient, k o, and the cell mass transfer coefficient, k cell are consistently higher in the presence of HBOC. In A, for high RBC saturation values and P 50,s Hb=10 Torr, k o is nearly equal to the case where there no HBOC is present. As S c Hb falls, k o increased nearly four-fold due to the delayed unloading of oxygen from the HBOC. This is also evident from the contribution of the average oxygen flux leaving the RBC surface towards the total flux entering the wall. The contribution of the RBC flux, λ (Eq. 7) decreased from 78% to 41% as S c Hb decreased from 0.5 to 0.1. When HBOC P 50,s Hb=29.3 Torr, the contribution of the RBC flux stays fairly constant at 60% between S c Hb=0.5 and 0.1, i.e. approximately equal contribution from both the RBC and the HBOC. Hence other parameters being constant, it would be advantageous to use an HBOC with high oxygen affinity when supplying oxygen to tissue regions located towards the end of the capillary, since they can carry the O 2 for a longer duration. HBOCs with lower oxygen affinity would deliver more oxygen to the arteriolar end of the capillary network and precapillary regions. However, these conclusions may be dependent on a number of factors, including O 2 consumption rate and inlet PO 2. A minimal change in tissue PO 2 distribution can be observed as P 50,s Hb value is increased from 29.3 to 50 Torr. The model predictions are in agreement with experimental data and observations dealing with the effect of O 2 affinity on oxygen deliveryCitation[[28]], Citation[[29]] suggesting the use of HBOCs with low P 50 to limit oxygen unloading in arterioles and to target regions with low PO 2. Mass transfer coefficient values shown in A are further supported by the low RBC flux values calculated at low RBC saturations.
The capillary and cell mass transfer coefficients rise monotonically for HBOC cooperativities of 1 and 2.2, while there exists a maximum value for 3.5. The RBC flux contribution decreased from 76% to 62% for n s=1 and increased from 50% to 70% for n s=3.5 as S c Hb decreased from 0.5 to 0.1. Combining these results with those in , we can conclude that other parameters being constant, HBOCs with a higher cooperativity deliver oxygen more uniformly, leading to a higher average tissue PO 2 compared to HBOCs with a cooperativity of 1. Despite the large variations in capillary mass transfer coefficient values for n s=3.5 over the entire range of RBC saturation values, HBOCs with higher cooperativity provide higher oxygenation levels under hypoxic conditions.
We have shown that mass transfer coefficients strongly depend on capillary hematocrit or lineal density, LD, for a range of LD=36–72 cells/mm; this range was based on measurements in the hamster retractor muscle. For comparison, hemodilution experiments under resting conditions in the hamster cremaster muscle have shown that a reduction in H sys from 0.57 to 0.18 leads to a corresponding decrease in cell density from 94 to 50 cells/mm.Citation[[30]]
Though there is a large variation in k o for different values of P 50,s Hb and n s, the overall intracapillary resistance is always lower in the presence of HBOCs. This is because the driving force for oxygen is smaller when HBOC is present in plasma, and, given that the luminal flux is constant for all cases, this in turn translates into a higher value of k o. The extent to which HBOCs improve oxygen transport largely depends on the properties and concentration.
The model predictions are in agreement with theoretical results obtained for small blood vessels without HBOC by Hellums et al. (1996).Citation[[2]] They showed that for vessels with diameter 5.5 μm, the mass transfer coefficient remains approximately constant over an RBC saturation range of 0.8 to 0.1. The mass transfer coefficient for the control case presented in is also nearly constant for the same range of RBC saturation values. The increase in the overall mass transfer coefficient with increasing cell spacing shown by Hellums et al. [1996]Citation[[2]] is consistent with the results in A and our previous work.Citation[[11]]
CONCLUSIONS
It should be noted that our simulations are limited to consumption remaining constant, i.e., hypoxic conditions are not considered. A detailed analysis of hypoxic conditions is of great importance and should be considered in the future. The present paper is a theoretical study on the effect of HBOCs on oxygen delivery in capillary-size vessels. The only existing theoretical model of oxygen transport with HBOC considers arteriolar size permeable tubes;Citation[[7]] the results are presented in terms of the mean Hb saturation vs. residence time in the tube and the corresponding mass transfer coefficients cannot be readily calculated from the results. Hence, this is the first paper to use a discrete cell model to compute oxygen transport in capillary size vessels in the presence of HBOCs and under conditions of low hematocrit. The mass transfer coefficients calculated in the study can be represented as functions of various model parameters, making it possible to use them in microvascular network calculations where the heterogeneous flow distribution is likely to cause the hematocrit and transport parameters to vary over the entire microvascular network. While the overall conclusion is that HBOCs enhance delivery of oxygen, there are additional findings that follow from our study and are summarized below.
The capillary and cell mass transfer coefficients, k o and kcell, are strong functions of both average RBC oxyhemoglobin saturation and hematocrit. It is a significant relationship that has not been reported before.
Under the conditions considered in this work, lower values of P50,sHb tend to delay oxygen delivery to the venular end of the capillary, while HBOCs with higher P50,sHb values unload more oxygen near the arteriolar end of the capillary, in agreement with previous experimental studies.Citation[[28]], Citation[[29]]
Tissue oxygenation improved progressively as HBOC concentration increased from 0 to 10 g/dl.
Under resting conditions the effect of convection and Hb-facilitated diffusion of O2 are not significant.
In the presence of HBOCs, substantial deviations from chemical equilibrium can significantly influence tissue PO2.
ACKNOWLEDGMENTS
This project was supported by the National Institutes of Health Grant HL-18292, American Heart Association Postdoctoral Fellow Grant, and the Johns Hopkins University Center for Advanced Transfusion Practices and Blood Research.
REFERENCES
- Alayash A. I. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants?. Nat. Biotechnol. 1999; 17(6)545–549
- Hellums J. D., Nair P. K., Huang N. S., Ohshima N. Simulation of intraluminal gas transport processes in the microcirculation. Ann. Biomed. Eng. 1996; 24(1)1–24
- Federspiel W. J., Popel A. S. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc. Res. 1986; 32(2)164–189
- Wang C. H., Popel A. S. Effect of red blood cell shape on oxygen transport in capillaries. Math. Biosci. 1993; 116(1)89–110
- Groebe K. An easy-to-use model for O 2 supply to red muscle. Validity of assumptions, sensitivity to errors in data. Biophys. J. 1995; 68(4)1246–1269
- Popel A. S. Theory of oxygen transport to tissue. Crit. Rev. Biomed. Eng. 1989; 17(3)257–321
- Page T. C., Light W. R., Hellums J. D. Prediction of microcirculatory oxygen transport by erythrocyte/hemoglobin solution mixtures. Microvasc. Res. 1998; 56(2)113–126
- Swain D. P., Pittman R. N. Oxygen exchange in the microcirculation of hamster retractor muscle. Am. J. Physiol. 1989; 256(1 Pt. 2)H247–H255
- Vovenko E. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pfluegers Arch. 1999; 437(4)617–623
- Honig C. R., Gayeski T. E. Precapillary O 2 loss and arteriovenous O 2 diffusion shunt are below limit of detection in myocardium. Adv. Exp. Med. Biol. 1989; 248: 591–599
- Eggleton C. D., Vadapalli A., Roy T. K., Popel A. S. Calculations of intracapillary oxygen tension distributions in muscle. Math. Biosci. 2000; 167(2)123–143
- Goldman D., Popel A. S. A computational study of the effect of capillary network anastomoses and tortuosity on oxygen transport. J. Theor. Biol. 2000; 206(2)181–194
- Bos C., Hoofd L., Oostendorp T. Reconsidering the effect of local plasma convection in a classical model of oxygen transport in capillaries. Microvasc. Res. 1996; 51(1)39–50
- Secomb T. W., Hsu R., Pries A. R. A model for red blood cell motion in glycocalyx-lined capillaries. Am. J. Physiol. 1998; 274(3 Pt. 2)H1016–H1022
- Ellsworth M. L., Popel A. S., Pittman R. N. Assessment and impact of heterogeneities of convective oxygen transport parameters in capillaries of striated muscle: experimental and theoretical. Microvasc. Res. 1988; 35(3)341–362
- Paletta L. E. Effect of Hemoglobin Solutions on Microvascular Oxygen Transport Following Acute Isovolemic Hemodilution. M.S. Thesis. Virginia Commonwealth University, Richmond 1997
- Honig C. R., Gayeski T. E. Correlation of O 2 transport on the micro and macro scale. Int. J. Microcirc.: Clin. Exp. 1982; 1(4)367–380
- Fronek K., Zweifach B. W. Pre- and postcapillary resistances in cat mesentery. Microvasc. Res. 1974; 7(3)351–361
- Zweifach B. W. Quantitative studies of microcirculatory structure and function. I. Analysis of pressure distribution in the terminal vascular bed in cat mesentery. Circ. Res. 1974; 34(6)843–857
- Monkos K. Viscometric study of human, bovine, equine and ovine haemoglobin in aqueous solution. Int. J. Biol. Macromol. 1994; 16(1)31–35
- Pries A. R., Secomb T. W., Gessner T., Sperandio M. B., Gross J. F., Gaehtgens P. Resistance to blood flow in microvessels in vivo. Circ. Res. 1994; 75(5)904–915
- Roy T. K., Popel A. S. Theoretical predictions of end-capillary PO 2 in muscles of athletic and nonathletic animals at VO 2 max. Am. J. Physiol. 1996; 271(2 Pt. 2)H721–H737
- Vadapalli A., Pittman R. N., Popel A. S. Estimating oxygen transport resistance of the microvascular wall. Am. J. Physiol.: Heart Circ. Physiol. 2000; 279(2)H657–H671
- Wang D., Kreutzer U., Chung Y., Jue T. Myoglobin and hemoglobin rotational diffusion in the cell. Biophys. J. 1997; 73(5)2764–2770
- Spaan J. A., Kreuzer F., van Wely F. K. Diffusion coefficients of oxygen and hemoglobin as obtained simultaneously from photometric determination of the oxygenation of layers of hemoglobin solutions. Pfluegers Arch. 1980; 384(3)241–251
- Nishide H., Chen X. S., Tsuchida E. Facilitated oxygen transport with modified and encapsulated hemoglobins across non-flowing solution membrane. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1997; 25(4)335–346
- Bouwer S. T., Hoofd L., Kreuzer F. Diffusion coefficients of oxygen and hemoglobin measured by facilitated oxygen diffusion through hemoglobin solutions. Biochim. Biophys. Acta 1997; 1338(1)127–136
- Sakai H., Tsai A. G., Rohlfs R. J., Hara H., Takeoka S., Tsuchida E., Intaglietta M. Microvascular responses to hemodilution with Hb vesicles as red blood cell substitutes: influence of O 2 affinity. Am. J. Physiol. 1999; 276(2 Pt. 2)H553–H562
- Intaglietta M. Microcirculatory basis for the design of artificial blood. Microcirculation 1999; 6(4)247–258
- Sarelius I. H. Microcirculation in striated muscle after acute reduction in systemic hematocrit. Respir. Physiol. 1989; 78(1)7–17
- Mahler M., Louy C., Homsher E., Peskoff A. Reappraisal of diffusion, solubility, and consumption of oxygen in frog skeletal muscle, with applications to muscle energy balance. J. Gen. Physiol. 1985; 86(1)105–134
- Bentley T. B., Meng H., Pittman R. N. Temperature dependence of oxygen diffusion and consumption in mammalian striated muscle. Am. J. Physiol. 1993; 264(6 Pt. 2)H1825–H1830
- Meng H., Bentley T. B., Pittman R. N. Myoglobin content of hamster skeletal muscles. J. Appl. Physiol. 1993; 74(5)2194–2197
- Jurgens K. D., Peters T., Gros G. Diffusivity of myoglobin in intact skeletal muscle cells. Proc. Natl. Acad. Sci. U. S. A. 1994; 91(9)3829–3833
- Respiration and Circulation, P. L. Altman, D. S. Dittmer. Fed. Am. Soc. Exp. Biol., Bethesda, MD 1971
- Goldstick T. K., Ciuryla V. T., Zuckerman L. Diffusion of oxygen in plasma and blood. Adv. Exp. Med. Biol. 1976; 75: 183–190
- Kayar S. R., Hoppeler H., Hudlicka E., Uhlmann E., Tyler K. R. Capillary dimensions and barrier thickness from capillaries to muscles. Acta Anat. 1987; 128: 370
- Liu C. Y., Eskin S. G., Hellums J. D. The oxygen permeability of cultured endothelial cell monolayers. Adv. Exp. Med. Biol. 1994; 345: 723–730
- Christoforides C., Laasberg L. H., Hedley-Whyte J. Effect of temperature on solubility of O 2 in human plasma. J. Appl. Physiol. 1969; 26(1)56–60
- Clark A., Jr., Federspiel W. J., Clark P. A., Cokelet G. R. Oxygen delivery from red cells. Biophys. J. 1985; 47(2 Pt. 1)171–181
- Stein J. C., Ellsworth M. L. Capillary oxygen transport during severe hypoxia: role of hemoglobin oxygen affinity. J. Appl. Physiol. 1993; 75(4)1601–1607
Appendix
MATHEMATICAL MODEL EQUATIONS
Mass Transport in the Tissue
In the tissue, the transport of oxygen takes place via diffusion of free O 2 and the facilitated diffusion of O 2 bound to myoglobin (Mb). The frame of reference is moving with the RBC, hence the mass transfer equation in the tissue has the convective term with velocity −v c. The mass transport equation is expressed as where P is the oxygen tension, α is the solubility coefficient of O 2, D o is the diffusion coefficient of O 2, D Mb is the diffusivity of Mb in the tissue, [Mb] is the concentration of myoglobin in the tissue, and M t is the O 2 consumption in the tissue, which is assumed to be constant for the entire tissue region.
Passive Diffusion in the Interstitium and the Capillary Wall
We assume that there is no extravasation of free Hb from the lumen. Thus, free oxygen is the only species diffusing through the interstitium and capillary wall, where α and D o are specific to the region, M=0 in the interstitium and M=M w inside the wall.
Mass Transport in Plasma
In the plasma, free oxygen dissociates from the HBOC. Bound O 2 in the HBOC is restricted to the plasma region. The oxygen tension and the oxyhemoglobin saturation in plasma, S s Hb, are governed by the transport equations
where S s Hb=[HbO 2]/[Hb] s, [HbO 2] is the molar concentration of the oxygenated HBOC in plasma, [Hb] s the total concentration and D Hb is the diffusivity of the HBOC in plasma. The last term represents the kinetic reaction between free O 2 and HBOC, with k s Hb representing the dissociation rate, P 50,s Hb the O 2 tension that corresponds to 50% saturation of the hemoglobin-based solution, and n s the Hill coefficient of the saturation curve. Both P50,s Hband ns in Eqs. A411 and A512 are parameters specific to the HBOC present in the plasma. In the absence of HBOC, one solves only Eq. A411 with [Hb]s=0.
Mass Transport in the Red Blood Cell
In the red blood cell, the free O 2 diffuses out to the plasma in the direction of the gradient while the oxyhemoglobin is restricted to the red blood cell. Similar to Eqs. A310 and A411, we formulate the transport equations for PO2 and fractional saturation of oxyhemoglobin in the RBC, ScHb:
where [Hb] c is the total concentration of Hb in the RBC; k c Hb is the kinetic dissociation rate constant; P 50,c Hb is the PO 2 that corresponds to 50% saturation of the hemoglobin; and n c is the corresponding Hill coefficient of the O 2 dissociation curve. The values of P 50,c Hb and n c are kept the same for all simulations. Note that since the frame of reference is moving with the RBC, there are no convective terms in Eqs. A512 and A613. The values of the model parameters used in the simulations for all the sub-regions in the computational domain are presented in .
Initial and Boundary Conditions
In order to solve the set of nonlinear partial differential Eqs. A1–A6 8-13, we need to prescribe initial and boundary conditions for the domain. The present model focuses on a specific section of the capillary containing several red blood cells. For a given segment with constant vascular wall and tissue oxygen consumption rates, the average flux leaving the capillary is constant by mass balance over all the cells. A Krogh-type solution is used to prescribe the initial and boundary conditions as follows. The simplified continuum transport equation for RBC–HBOC mixture is formulated for the capillary with a mass transport coefficient, ko, at the lumen of the capillary wall. This set of equations can be solved if ko is known in order to determine the distributions of PO 2 and SO 2 in the capillary and tissue. Initially, a constant value of k o is chosen based on previous calculations.Citation[[11]] The obtained Krogh-type solution is used as the initial condition for the moving region containing three RBCs and also as the boundary conditions as described below. From the numerical solution of Eqs. A1–A68-13, the function ko(z) is calculated. This function is substituted into the Krogh-type solution, and the process continues iteratively until the convergence of ko(z) is achieved. For the present model parameters, two iterations were sufficient to achieve convergence.
To obtain the Krogh-type solution (denoted by subscript k), we express O 2 flux leaving the capillary wall per unit length (FO 2) as where
is the average plasma velocity, S ck(P ck) and S sk(P sk) are the mixing-cup oxyhemoglobin saturations in the cell and plasma phases, respectively, with P ck and P sk the PO 2 values in the red blood cell and plasma, respectively. We assume that P ck=P sk, and we neglect dissolved oxygen while solving Eq. A714 since its concentration is much smaller than that of oxygen bound to the heme protein in both the RBC and plasma. Note that Eq. A815 is written in the frame of reference of the tissue unlike Eqs. A1–A68-13 where the frame of reference is located on the RBC. The O2 balance is completed by equating the longitudinal loss in O2 in Eq. A714 with the flux leaving the capillary wall in the radial direction
At the plasma-wall interface the boundary condition is where
is the O 2 flux at the wall calculated from Eq. A815 with known values of tissue and wall O2 consumption rates, k o(z) is the initial value of the intracapillary mass transfer coefficient as defined in Eq. 5, and P wk is the PO 2 at the wall computed using Eq. A916. P ck is calculated from Eqs. A7–A914-16 and is then used with the value of Pwk to calculate the O2 distribution in the radial direction for the wall, interstitium and tissue by solving
where P k is the PO 2 outside the capillary, D o is O 2 diffusivity, α is the O 2 solubility coefficient, and M is the O 2 consumption rate of either the tissue or the vascular wall as listed in . It was possible to obtain an analytical solution for Eqs. A7–A1014-17 when no HBOC is present in the plasma and in the case where the HBOC cooperativity in the plasma, n s, is equal to that of the RBC Hb cooperativity, n c. For the case when n s≠n c, Eqs. A7–A1014-17 were solved numerically.
The Krogh-type solution is also used for prescribing the boundary conditions at each end of the domain. The coordinate system of the domain was centered about the middle cell with the leftmost node (the trailing edge) at z=−(3L/2) and the rightmost node (the leading edge) at z=−(3L/2). The boundary condition at the trailing edge was
The boundary condition at the leading edge (i.e. z=3L/2) is the same except for a negative sign on the right hand side of Eqs. A11–A13 18-20. Because of the condition of axisymmetry
At the outer edge of the tissue
The above set of boundary conditions satisfied the mass balance in the tissue region with the average flux leaving from all the cells being equal to the consumption rate of the tissue. The capillary mass transfer coefficient k o(z) was obtained by solving Eqs. A1–A68-13 iteratively with value of ko(z) computed at the end of each iteration using Eq. 5 being used in Eq. A9 16 for the subsequent iteration.
![Figure 4. (A) Comparison of k o for different values of HBOC oxygen cooperatives. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=2.2, H c=0.2, [Hb] s=7 g/dl. (B) Comparison of k cell for different values of HBOC oxygen cooperatives. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=2.2, H c=0.2, [Hb] s=7 g/dl.](/cms/asset/2a3deeb3-ab94-45b3-bf1f-a613040c0e4b/ianb19_a_11116809_uf0004_b.gif)
![Figure 9. (A) Comparison of k o for different values of hematocrits. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=n s=2.2, [Hb] s=7 g/dl. (B) Comparison of k cell for different values of hematocrits. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=n s=2.2, [Hb] s=7 g/dl.](/cms/asset/d3fa156a-9946-45c1-a0e2-17a3ffeacda8/ianb19_a_11116809_uf0009_b.gif)
![Figure 10. Comparison of kinetic parameter values with and without HBOC. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=n s=2.2, [Hb] s=7 g/dl.](/cms/asset/198ea137-9f16-486f-8e96-f8c0362a08f4/ianb19_a_11116809_uf0010_b.gif)
![Figure 11. Comparison of overall mass transfer coefficient for different HBOC diffusion coefficients. P 50,c Hb=P 50,s Hb=29.3 Torr, n c=n s=2.2, [Hb] s=7 g/dl.](/cms/asset/56c7b240-bd72-4e47-b205-2deb97d55a6e/ianb19_a_11116809_uf0011_b.gif)