Abstract
Approaches using immobilized biological materials are very promising for application in different branches of the food industry, especially in the production of fermented beverages. Materials tested by our team for the process of entrapment belong to the family of charged polysaccharides able to form beaded hydrogels by ionotropic gelation (e.g. alginate, pectate, κ-carrageenan) and synthetic polymers (e.g. polyvinyl alcohol) forming bead- and lens-shaped hydrogels by thermal sol/gel transition. Concentration of a gel, conditions and instrumentation of gelation process, bead and size distribution, porosity, diffusion properties, mechanical, storage and operational stability, and many other parameters were followed and optimized. Our work has been oriented especially to practical applications of immobilized cells. Brewing yeast cells were successfully immobilized by entrapment materials and used in a process of batch and continual production of beer, including primary and secondary fermentation of wort. Other applications include continual production of ethanol by fermentation of different saccharide substrates (molasses, glucose syrup, wheat hydrolysate), mead and non-alcoholic beverages production.
| ABBREVIATIONS | ||
| APA | = | pectate prepared from apple pectin by alkaline hydrolysis |
| APE | = | pectate prepared from apple pectin by enzymatic hydrolysis |
| CAG | = | calcium alginate gel |
| CPA | = | pectate prepared from citrus pectin by alkaline hydrolysis |
| CPE | = | pectate prepared from citrus pectin by enzymatic hydrolysis |
| CPG | = | calcium pectate gel |
| GA | = | glutaraldehyde |
| LAB | = | lactic acid bacteria |
| PEI | = | poly(ethyleneimine) |
| PVAL | = | poly(vinylalcohol) |
| SE-HPLC | = | size-exclusion high-performance liquid chromatography |
| TCA | = | tricarboxylic acid cycle |
INTRODUCTION
New immobilization materials and techniques are being developed, making the immobilized biocatalysts more accessible to industrial applications. Entrapment of cells into gel matrices has by far the largest significance of all immobilization techniques for whole cells.
In the past, the main targets of process development and engineering were in the field of reactor development, reactor modification and optimization of special biotechnological applications. Despite those efforts, no substantial success was achieved to introduce biotechnological processes, which were economically comparable to chemical processes, if some minor exceptions were neglected. This is mainly caused by the fact that the product of interest is highly diluted in an aqueous solution, therefore downstream processing costs become economically limiting factors.Citation[[4]] Not long ago, no entrapment process had existed which allowed the production of small (<1 mm), uniformly distributed beads in high production rates from high viscous polymer solutions.Citation[[5]] In order to resolve this problem, the JetCutting method as a new entrapment/immobilization technique has been developed.Citation[[6]]
Pectins from the soft tissue of higher plants, in many ways the land-based counterpart of alginate in sea plants, have yet found only negligible applications in biochemical technology. Despite this lack of interest, evidence of their unique properties exists, favouring especially pectates (d.e. <3%) as the most attractive material within the family of pectin biopolymers.Citation[7-9]
Like alginate, ionotropic gelation of pectate is simple and inexpensive. It also provides mild and physiological conditions for cell entrapment. Calcium pectate gel (CPG) is not only an alternative to calcium alginate gel (CAG); various advantages of CPG over CAG have been recognized. In particular, CPG beads are less sensitive to ions and chemical agents, which destroy CAG beads; the stability constant of calcium pectate is almost one order of magnitude higher than that of calcium alginate. However, for some applications, CPG beads must be stabilized and hardened when used continuously. Thus, preformed beads were treated with polyethylenimine (PEI) followed by glutaraldehyde (GA) treatment. In this way, a layer was formed which increased substantially the stability of the CPG beads towards Ca 2+-complexing reagents, lowered pH, as well as mechanical stress. Stabilization and hardening of CPG beads did not substantially change their morphologic properties, such as the porosity, pore size distribution, size-exclusion limit or plasticity. Entrapped cells reduced the network density of CPG beads determined from mechanical measurements and the resistance of CPG material to deformation, even when stabilized with PEI and GA. With respect to viability and toxicity, only short-term exposure of GA was tested; the effect of GA on the stability of CPG beads was perceptible.Citation[[8]]
The deesterified non-depolymerized form of pectins, i.e. pectates, salts of poly-D-galacturonic acid is, therefore, a very suitable form of the precursor for cell/biocatalyst entrapment by ionotropic gelationCitation[7-8] The above-mentioned pectates have not been commercially available as yet, although the world production and unit price of pectins were comparable to alginatesCitation[[10]] and despite the fact that the pectin is a natural product with a long record of safety, applied particularly in food chemistry, dairy products and pharmaceuticalsCitation[11-12] referred in updated monographs.Citation[13-14] This review article summarizes the state of knowledge about the preparation of pectates suitable for entrapment of whole living cells and their application in food industry.Citation[[15]]
For technical use highly elastic and stable hydrogels like polyvinyl alcohol (PVAL) are favoured. According to a new method, the gelation to the PVAL hydrogel (10% w/w PVAL and 90% water) occurs at room temperature.Citation[[16]] This new method works without the cost-intensive freezing and thawing procedure, which complicates the technical use of PVAL hydrogels as matrices for cell/biocatalyst immobilization. Particles could be obtained in bead or lens (LentiKats) shape.Citation[[16]]
Since 1960, the process of immobilization of enzymes and later whole cells has shown a great expansion, and many practical applications have been suggested. The main advantage which led to the research is that the price of the treated product can be lowered, since the preparation is used continuously with enzymes in higher concentrations. However, the purification of enzymes is onerous, so it was of interest to use whole viable microorganisms with high enzymatic activities. Later, many authors confirmed this possibility and a real explosion of research has taken place; the applications of this technology are very promising in many fields of food industry.Citation[[17]]
RESULTS AND DISCUSSION
Macromolecular Characteristics of Pectate Gel Precursors
As already mentioned in the introductory section, pectates suitable for preparation of CPG are, so far, commercially not available, what might be due to the deesterification mode of pectin. This lack is documented in by the deformability, intrinsic viscosity η and M w values of laboratory-prepared apple and citrus pectate samples obtained by alkali-catalyzed deesterification. The values listed indicate that a massive depolymerization took place during deesterification. It follows that commercial pectates were produced by alkaline deesterification accompanied by depolymerization.
Table 1. Macromolecular Characteristics of Commercial and Laboratory-Prepared Samples of Pectate Gel Bead Precursors Related to Mechanical Properties of Calcium Alginate Gel Beads
compares macromolecular characterictics of commercial alginates and laboratory-prepared pectates as produced by enzymic deesterification. Pectates and alginates of the closest content of gel-forming uronic acids (D-galacturonic and L-guluronic acids corresponding to pectates and alginates, respectively) were selected in the first screening. In contrast to the precedent papersCitation[[7]], Citation[[15]], Citation[18-20] (alginic acid, sodium salt of Fluka, Janssen Chimica, etc.), the reference alginates described in this paper are represented by commercial preparation of sodium alginate (Protanal-produced by Protan Biopolymers, Drammen) with a known content of L-guluronic acid (Anonymous). Also further fundamental macromolecular characterictics data of this material are reportedCitation[21-22] and actualized continuously;Citation[[7]], Citation[[21]] the Protanal grade preparations, recommended for immobilization and encapsulation of a large number of different cells including bacteria, yeasts, molds, algae, blue/green algae, plant and animal cells, are of high purity.Citation[[23]] This is also the reason why these commercial preparations are gaining dominant position as alginate carriers of immobilized biosystems.Citation[[21]]
Table 2. Macromolecular Characteristics of Polysaccharide Gel Precursor
It seems, however, that the functional properties of each of ionotropically formed polyuronate gel (see ) are governed by macromolecular properties of the polysaccharide gel precursors.Citation[[7]], Citation[[15]], Citation[[20]] The following essential macromolecular properties of the respective precursors have to be checked at least when comparing suitability of both materials (CPG and CAG) for cell entrapment: average molecular weight (or, if necessary, limiting viscosity number,Citation[[15]], Citation[[20]] Staudinger index,Citation[[18]] calcium-linking uronate unit (D-galacturonate in pectate and L-guluronate in alginate) contentCitation[[7]], Citation[[15]], Citation[[20]] and, in the case of CPG, degree of esterification (d.e. <3%).Citation[[7]], Citation[[15]], Citation[[18]] The necessary data either fully absentedCitation[24-27] or were incomplete,Citation[[18]], Citation[28-29] or the macromolecular properties of polyuronate gel precursors (pectate, alginate) were insufficiently compatible in papersCitation[[7]], Citation[[15]], Citation[[20]] published so far. This information lack resulted then in incorrect general interpretation of results of comparative studies dealing with functional properties of calcium polyuronate gels. Therefore, this paper warns of the possible misinterpretation and recommends ways to avoid it.
Table 3. Macromolecular Characteristics of Polysaccharide Gel Precursor
In macromolecular characteristics of laboratory-prepared pectates are presented. Both pectates of citrus (CPA) and apple (APA) origins were prepared by alkaline deesterification in heterogeneous phase under strictly controlled conditions at which the minimal degradation of pectin backbone occurred. In this way prepared potassium pectates had similar molecular properties as had pectates produced by enzymatic deesterification (). Consequently, the mechanical characteristics of calcium pectate gel beads obtained from alkali deesterified pectates were comparable to those of calcium pectate gel beads prepared from enzymatically deesterified pectate which we consider the reference material as the enzymatic deesterification using pectinesterase proceeds as specific reaction without almost any depolymerization process. Moreover, the controlled alkaline deesterification is more advantageous from the stand-point of economy and simplicity.
Viscosity and Mechanical Characteristics of Calcium Polyuronate Gel Beads
In order to specify physical properties of the polyuronate gels more in detail, measurements of viscosity and mechanical parameters have been carried out. In , one can see that the gel viscosities differ two orders of magnitude depending on the type and concentration of the gel. The alginate gels possess very high viscosities, despite lower concentrations applied than those of the pectates and the pectinates. In contrast, the pectates, even with a high gel concentration, have the lowest viscosities.
Figure 1. Viscosity curves as a function of the shear rate γ. The measurements were done at 25°C using the rotational viscometer VT550 (Haake, Germany). Last digit in legends expresses the gel concentration in % (w/w): A10–Protanal LF 10/60; A20–Protanal LF 20/60 Pronova, Drammen, Norway; FAL–pectinate type 1, Federal Agricultural Research Centre, Braunschweig, Germany; APA–pectate type 4, Slovak Academy of Sciences, Bratislava, Slovak Republic. ▪–A102, •–A103, ▴–A201, ▾–A202, ♦–A203, ◂–FAL5, ▸–FAL8, ⧫–APA5, *–APA8.
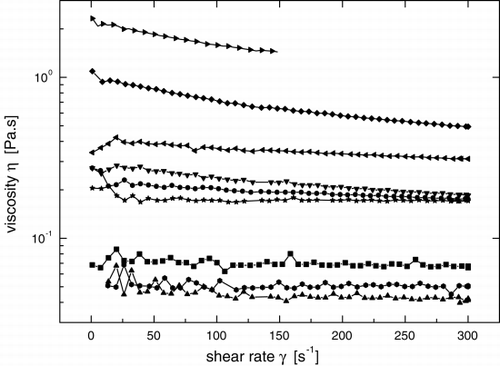
It is noticeable that viscosity curves of alginates and pectinates show a declined course that is typical for the pseudoplastic fluid behavior (Figure). For that reason, flow curves were evaluated according to the Ostwald de Waele model using two rheological parameters—the consistence index K and the flow index n: where τ is the shear stress and γ is the shear rate.
The results in show that alginates as well as pectinates exhibited well-marked, non-Newtonian, pseudoplastic behaviour (the flow index n was significantly lower than 1). Surprisingly, the pectate gels, even at the highest gel concentrations, represent a lower-viscosity Newtonian fluid (flow index is either equal or very close to 1). From practical point of view, when Jet-Cutting Technology is used for a preparation of the alginate or pectinate gel beads, it can be advantageous to apply higher shear rates in a nozzle with flowing gel fluid. Thus, it allows to prepare the beads with higher gel concentration. However, in the case of pectates, any shear rate applied will not change the gel viscosity.Citation[[30]]
Table 4. Mechanical Characteristics of Calcium Polysaccharide (Polyuronate) Gel Beads Prepared by Single-Nozzle JetCutter Device (Prüße et al., 1988) and Measured by Device According to Washausen
Actually, for the comparison, the polyuronate gels of the same concentrations should be tested. However, alginate beads have to be prepared technologically at a lower 2–3% (w/v) concentration than pectates (5–10% w/v) due to their high viscosity. In the mentioned range of gel concentrations, the Jet-Cutting technology offers particles of uniform form and size. As a result, the alginate beads possess much smaller mechanical parameters in comparison with the particles prepared of more viscous solutions of pectates (see Tables and ). Pectates have the highest deformation stability of gel beads. Moreover, in our previous review article it was shownCitation[[8]] that also hardening procedureCitation[[20]], Citation[[31]] significantly influences mechanical parameters of these gels.
It can be concluded that the pectate gel represents a lower-viscosity Newtonian fluid that allows the preparation of gel beads of more acceptable (smaller) diameter at high gel concentrations. The low viscosity in conjunction with high mechanical stability of beads and low intrinsic viscosity are favorable properties which make the calcium pectate gel an attractive immobilization material for many biotechnological applications.
Table 5. Rheological Parameters K (Consistence Index) and n (Flow Index) Evaluated According to the Ostwald de Waele Model for Pseudoplastic Liquids− τs= Kγ n, Where τ is the Shear Stress and γ is the Shear Rate (Viscosity Measurements Were Done at 25°C (Temperature at Which Bead Preparation Is Done) and 35°C (Many Fermentations Are Done at This Temperature) Using Rotational Viscometer VT550 (Haake, Germany))
Biotechnological Processes Using Pectate-Immobilized Cells
Our recent research using immobilized biocatalysts has been focused especially on fermentation processes. We have specialized in production of both alcoholic and non-alcoholic beer, fermented fruit and vegetable juices, fuel and food ethanol and mead. Lots of these processes have been improved by continuous operation mode using immobilized cells, where processing time was significantly shortened and analytical and desirable flavor characteristics of product were maintained.Citation[[32]]
In each of the cases, calcium pectate was used as an immobilization material. But we have tested calcium alginate, κ-carrageenan and polyvinyl alcohol as well. The results show that calcium pectate gels (CPG) are the most suitable material because of their mechanical stability and acceptability in food industry.Citation[[33]]
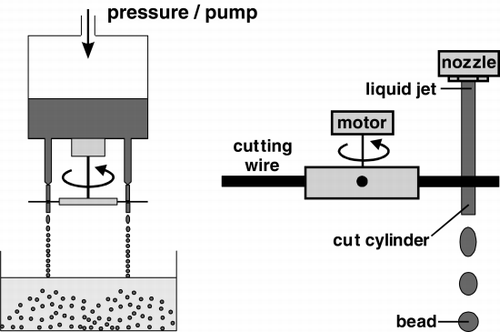
The immobilizates have been prepared in two ways. The former used a simple laboratory device for preparation of smaller amounts of the gel beads. Beads with varying mean diameter can be obtained (0.5–3 mm) depending on the gel concentration (). The latter involved preparation of gel beads by a novel “jet-cutting” system (). It is much more effective and is able to produce several liters of immobilizates per hour. Also, the bead size is more uniform.
Figure 2. Simple laboratory multi-nozzle device for gel bead production. The solution of potassium pectate containing the cell suspension is pushed through the needle and dropped into the CaCl 2 solution.
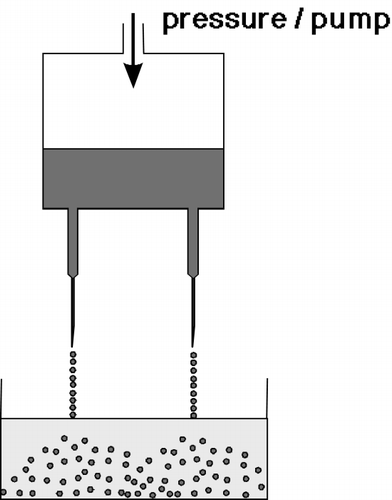
Figure 3. Sophisticated “jet-cutting” system for production of gel immobilizates. The beam of potassium pectate solution containing the cell suspension running from the nozzle is cut by single- (Prüße et al., 1998) or multi-wire cutting rotary device.
Recently we have begun to test the novel immobilization material PVAL (polyvinyl alcohol), which uses the sol–gel transition for gelating. It is characterized by good mechanical stability, and the mass transport is improved too, because the immobilizates are of lens shape. It helped to improve operational stability of fermentation processes and in some cases made the process more effective.
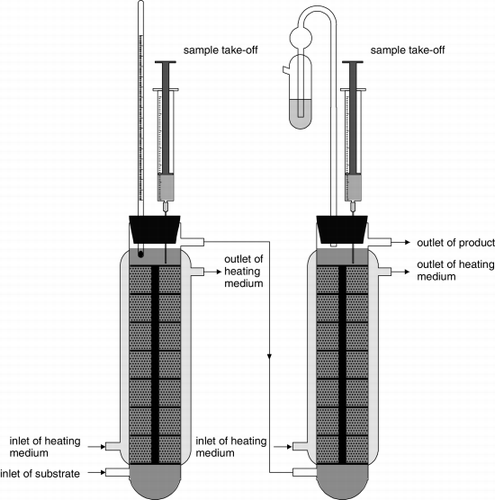
Continuous Primary and Secondary Beer Fermentation
Continuous production of beer has been the subject of research for many years. In the brewing industry during the 1980s there was a great increase in immobilized yeast technology research. The principle of this technology is to produce high yeast cell density in order to rapidly ferment wort and/or mature beer. The most widespread cell immobilization technique is entrapment in a matrix or adherence of cells to the surface of a large number of sintered or porous materials.Citation[[34]] Typical examples of polymers used for entrapment of cells include alginates, κ-carrageenan, polyacrylamide or gelatin. The mechanical strength of the gel matrix is important for minimizing gel splitting or stripping of yeast cells from the matrix due to the evolution of CO 2 by the immobilized yeast during fermentation.
It is necessary to obtain sufficient mass transfer of won nutrients to the cells and mass transfer of fermentation products away from the cells. Therefore, immobilized yeast reactor design plays an important role in the successful application of immobilized brewing yeast cell systems. We have chosen a gas-lift bioreactor system, which has not been described for continuous primary wort fermentation yet and has been compared with multistage fluidized bed bioreactor system using calcium pectate gel as a matrix for entrapment of brewing yeasts S. cerevisiae W96.Citation[35-36] The beer produced in our gas-lift bioreactor system was of excellent quality with a composition and analytical parameters similar to that of beer produced by classical fermentation.Citation[[37]]
Immobilized yeast in a two-stage reactor system was applied to both primary and secondary laboratory fermentations of wort. The first stage, the primary fermentation process, was carried out in an up-flow gas-lift bioreactor, and in the second stage, maturation was processed in column reactors consisting of eight packed-beds filled with both yeasts entrapped in three different polysaccharide hydrogels and with free yeast in parallel. The residence time for the two-stage immobilized system varied from 74 to 108 h compared to up to two weeks in case of traditional beer production. Yeasts immobilized in PVAL provided excellent results too.Citation[[38]]
Continuous Production of Non-Alcoholic Beer
The production of non-alcoholic beer (containing less than 0.5% alcohol) is supposed to be a long-time problem in brewing industry. The need of method for non-alcoholic beer production is given by both legislative and health reasons. But the organoleptic properties of this kind of beer have to be comparable to those of beer produced by conventional methods. In the production of non-alcoholic beer, two main concepts are applied. The former is conducting the fermentation process to obtain minimal alcohol content, which is under concentration of 0.5%, known as “stop-fermentation.” The latter is separation of alcohol from classical beer using membrane processes (including dialysis and reverse osmosis) or its removal by distillation or vacuum evaporation.Citation[[39]] However, these approaches require special equipment for alcohol removing, which results in higher running costs. In addition, they may cause changes in flavor and taste, which is often watery.
The non-alcoholic beer, due to higher concentration of sugars and almost no alcohol, is much more sensitive to microbial contamination than normal alcoholic beers. In order to avoid this risk, pH value of wort must be decreased. The simplest way is just to add organic acid to the wort medium, where lactic acid is preferred. Then, Back et al.Citation[[40]] suggested a process for non-alcoholic beer production involving pretreatment of wort with lactic acid bacteria (LAB) fermentation. The produced lactic acid increases stability of wort and makes it less sensitive. But, the activity and growth of LAB is usually very difficult to control and the pretreatment step itself is relatively slow. Now, another way of non-alcoholic beer production can be applied, which involves usage of genetically manipulated strains of yeast.
Nuclear mutants of Saccharomyces cerevisiae used in this work are defective in synthesis of tricarboxylic acid (TCA) cycle enzymes, i.e. fumarase or 2-oxoglutarate dehydrogenase.Citation[41-42] Because of lack of TCA cycle enzyme activity, these strains produce minimal amounts of ethanol and, therefore, can be used for the production of alcohol-free beverages based on wort. The mutant strains were able to produce lactic acid in concentration up to 0.64 g.l −1, which together with other acids (total concentration was almost 1.4 g.l −1), resulted in decrease of pH to the values between 2.01 and 3.25. The total fermentation time took, depending on the fermentation mode, from 15 to 91 h. It means that the conventional process using LAB can be significantly simplified and shortened by use of the mutants of S. cerevisiae.
As data have shown, the immobilization process together with continual mode of fermentation is able to significantly reduce the fermentation time. Thus, the immobilized cells in a continuous fermentation are the most appropriate. The products have been tested by several tasters, and most of them considered the beers produced by these two strains to be comparable to those produced by a classical technology.Citation[[43]]
Production of Fermented Fruit and Vegetable Juices
The same cells, defected in TCA enzyme synthesis, were used in the production of fermented fruit and vegetable juices. Particularly fruit juices low in acid content are extremely appropriate for this kind of processing. During the process, the organic acids (lactic, fumaric, succinic) are produced in the fermentation medium. As the sugar content decreases, the acid concentration increases. Several fruit (pear, red melon, yellow melon, grapes) and vegetable (potato, carrot) juices were tested. Produced beverages were sensorially acceptable and due to low pH (around 2.5) were microbiologically stable without need to add artificial stabilizers.
Production of Ethanol and Mead Using Immobilized Yeast Cells
Other possible applications of immobilized biosystems are, i.e., production of ethanol or mead. Fermentation of molasses or starch hydrolyzate was used for ethanol production for both food and fuel purposes. There were batch and continuous modes examined, where the continuous mode exhibited higher operational stability. In the continuous mode, gas-lift and packed-bed bioreactor arrangements have been tested and compared. The experiments were carried out in a three-column system with decreasing fermentation temperature, which was supposed to compensate the increasing ethanol stress. The results show that the gas-lift arrangement is more effective, probably due to better diffusion parameters.
The latest experiments try to develop a continuous system for mead production. Mead is a traditional Slavic and Celtic alcoholic beverage containing 8–18% ethanol. It is prepared by fermentation of honey solution flavored by herbs and spices. This system allowed to shorten the primary fermentation time from several weeks (classical method) to 5–7 days (method using immobilized cells). Also, the continuous two-column system has been tested and optimized (). The results show that a bead/medium-volume ratio of 1:3 and fermentation temperature of 30°C provide the overall ethanol production rate of 5.7 g.l −1.h −1 with residence time of 24.5 h. The system was stable for 10 days without significant decrease in fermentation activityCitation[[44]]
CONCLUSION
Calcium pectate gel (CPG) appears to be a good alternative to calcium alginate gel (CAG), which is probably the most used material for immobilization of biocatalysts by entrapment. Molecular, morphologic, diffusional properties of CPG and CAG beads are very similar; however, CPG beads are less sensitive to chelating anions and chemical agents. Consequently, both stability constant of calcium pectate and mechanical stability of CPG-entrapped permeabilized cells are considerably better.
The immobilizates prepared like this can improve many fermentation processes. In many cases the continual process can be introduced, which is often much more effective than conventional batch process. In addition, the immobilization offers higher concentration of cells in the systems and hence higher production yields and rates.
ACKNOWLEDGMENTS
The authors (PG, JN) thank Prof. K.D. Vorlop, Dr. B. Willke and Dr. U. Prüsse from Institute für Technologie, FAL Braunschweig (FRG), for the possibility to use Jet-Cutter and mechanical test devices as well as corresponding schematic drawings.
REFERENCES
- Willaert R. G., Baron G. V. Gel entrapment and micro-encapsulation: methods, applications and engineering principles. Rev. Chem. Eng. 1996; 12: 1–205
- Jen A. C., Wake M. C., Mikos A. G. Review: Hydrogels for cell immobilization. Biotechnol. Bioeng. 1996; 50: 357–364
- Groboillot A., Boadi D. K., Poncelet D., Neufeld R. J. Immobilization of cells for application in the food industry. Crit. Rev. Biotechnol. 1994; 14: 75–107
- Gerbsch N., Buchholz R. New processes and actual trends in biotechnology. FEMS Microbiol. Rev. 1995; 16: 259–269
- Poncelet D., Neufeld R. J. Fundamentals of Dispersion in Encapsulation Technology. Progress in Biotechnology. Immobilized Cells: Basics and Applications, R. H. Wijffels, R. M. Buitelaar, C. Bucke, J. Tramper. Elsevier Science BV, Amsterdam 1996; Vol. 11: 47–54
- Prüße U., Fox B., Kirchhoff M., Bruske F., Breford J., Vorlop K. D. The Jet-Cutting method as a new immobilization technique. Biotechnol. Tech. 1998; 12: 105–108
- Gemeiner P., Rexová-Benková Ĺ., Švec F., Norrlöw O. Natural and Synthetic Carriers Suitable for Immobilization of Viable Cells; Active Organelles and Molecules. Immobilized Biosystems/Theory and Practical Applications, I. A. Veliky, R.J. C. McLean. Blackie Academic Professional, GlasgowLondon 1994; 1–128, An Imprint of Chapman Hall
- Gemeiner P., Nahálka J., Vikartovská A., Nahálková J., Tomáška M., Šturdík E., Markovič O., Malovíková A., Zatková I., Ilavský M. Calcium Pectate Gel Could be a Better Alternative to Calcium Alginate Gel in Multiple Applications of Immobilized Cells. Progress in Biotechnology. Immobilized Cells: Basics and Applications, R. H. Wijffels, R. M. Buitelaar, C. Bucke, J. Tramper. Elsevier Science BV, Amsterdam 1996; Vol. 11: 76–83
- http://www.genialab.de/inventory/
- Yalpani M., Sanford P. A. Commercial Polysaccharides: Recent Trends and Developments. Industrial Polysaccharides. Genetic Engineering; Structure/Property Relations and Applications. Progress in Biotechnology, M. Yalpani. Elsevier Science Publishers, Amsterdam 1987; Vol. 3: 311–335
- Thakur B. R., Singh R. K., Handa A. K. Chemistry and uses of pectin—a review. Crit. Rev. Food Sci. Nutr. 1997; 37: 47–73
- Walter R. H. The Chemistry and Technology of Pectin. Academic Press, New York 1991
- Barbotin J. N., Saucedo J.E. N. Bioencapsulation of Living Cells by Entrapment in Polysaccharide Gels. Polysaccharides: Structural Diversity and Functional Versatility, S. Dumitriu. Marcel Dekker, New York 1998; 749–774
- Dumitriu S., Chornet E. Polysaccharides as Support for Enzymes and Cell Immobilization. Polysaccharides: Structural Diversity and Functional Versatility, S. Dumitriu. Marcel Dekker, New York 1998; 629–748
- Kurillová Ĺ., Gemeiner P., Ilavský M., Štefuca V., Polakovič M., Welwardová A., Tóth D. Calcium pectate gel beads for cell entrapment. 4. Properties of stabilized and hardened calcium pectate gel beads with and without cells. Biotechnol. Appl. Biochem. 1992; 16: 236–251
- Jekel M., Buhr A., Willke T., Vorlop K. D. Immobilization of biocatalysts in LentiKats. Chem. Eng. Technol. 1998; 21: 275–278
- Diviès C. On the Utilization of Entrapped Microorganisms in the Industry of Fermented Beverage Production. Biotechnology Applications in Beverage Production, C. Cantarelli, G. Lanzarini. Elsevier Applied Science, London 1989; 153–168
- Berger R., Rühlemann I. Stable ionotropic gel for cell immobilization using high molecular weight pectic acid. Acta Biotechnol. 1988; 8: 401–405
- Gemeiner P., Kurillová Ĺ., Malovíková A., Tóth D., Tomašovičová D. Properties of spherical calcium pectate and alginate gels and their use in diffusion chromatography; solids separations and immobilization of enzymes and cells. Folia Microbiol. (Prague) 1989; 34: 214–227
- Gemeiner P., Kurillová Ĺ., Markovič O., Malovíková A., Uhrín D., Ilavský M., Štefuca V., Polakovič M., Báleš V. Calcium pectate gel beads for cell entrapment. 3. Physical properties of calcium pectate and calcium alginate gel beads. Biotechnol. Appl. Biochem. 1991; 13: 335–345
- Martinsen A., Skjåk-Braek G., Smidsrød O., Zanetti F., Paoletti S. Comparison of different methods for determination of molecular weight and molecular weight distribution of alginates. Carbohydr. Polym. 1991; 15: 171–193
- Smidsrød O., Skjåk-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990; 8: 71–78
- Anonymous. Technical Information-Alginates. Protan Biopolymers. Drammen, Norway 1991
- Montes M. C., Magana P. I. Δ′-Dehydrogenation of steroids by Arthrobacter simplex immobilized in calcium polygalacturonate beads. J. Ind. Microbiol. 1991; 8: 259–264
- Navarro A., Rubio M. C., Callieri D.A. S. Production of ethanol by yeasts immobilized in pectin. Eur. J. Appl. Microbiol. Biotechnol. 1983; 17: 148–151
- Navarro A., Marangoni H., Magana Plaza I., Callieri D. Horizontal reactor for the continuous production of ethanol by yeasts immobilized in pectin. Biotechnol. Lett. 1984; 7: 465–470
- Pras N., Hesselink P.G. M., Tusscher J. T., Malinré T. M. Kinetic aspects of the bioconversion of L-tyrosine into L-DOPA by cells of Mucuna pruriens L. entrapped in different matrices. Biotechnol. Bioeng. 1989; 34: 214–222
- Richter K., Rühlemann I., Berger R. High-performance fermentation with lactic acid bacteria entrapped in pectate gel immobilizates with enhanced lactate formation activity. Acta Biotechnol. 1991; 11: 229–241
- Rühlemann I., Richter K., Berger R. Ethanolic fermentation with Saccharomyces cerevisiae cells immobilized in pectate gel. Acta Biotechnol. 1990; 10: 55–61
- Navrátil M., Gemeiner P., Nahálka M., Klein J., Malovíková A., Šturdík E. Structure, Function, Properties of Biopolymers in Relation with Bioencapsulation. Proceedings of COST 840 Workshop, EspooFinland, December, 8–102000
- Tomáška M., Gemeiner P., Materlín I., Šturdík E., Handríková G. Calcium pectate gel beads for cell entrapment: a study on the stability of Kluyveromyces marxianus whole-cell lactase entrapped in hardened calcium pectate and calcium alginate gel beads. Biotechnol. Appl. Biochem. 1995; 21: 347–356
- Šmogrovičová D., Dömény Z. Beer volatile by-products formation at different fermentation temperature using immobilised yeasts. Process Biochem. 1999; 34: 785–794
- Navrátil M., Gemeiner P., Šturdík E., Dömény Z., Šmogrovičová D., Antalová Z. Fermented beverages produced by yeast cells entrapped in ionotropic hydrogels of polysaccharide nature. Minerva Biotecnol. 2000; 12: 337–344
- Masschelein C. A., Ryder D. S., Simon J. P. Immobilized cell technology in beer production. Crit. Rev. Biotechnol. 1994; 14: 155–177
- Šmogrovičová D., Dömény Z., Gemeiner P., Malovíková A., Šturdík E. Reactors for continuous primary beer fermentation using immobilised yeast. Biotechnol. Tech. 1997; 11: 261–264
- Dömény Z., Šmogrovičová D., Gemeiner P., Šturdík E., Malovíková A., Pátková J. Continuous secondary fermentation using immobilised yeast. Biotechnol. Lett. 1998; 20: 1041–1045
- Šmogrovičová D., Dömény Z., Švitel J. Effect of immobilised cell concentration on beer quality in continuous fermentation. Food Biotechnol. 1998; 12: 123–137
- Šmogrovičová D., Dömény Z., Navrátil M., Dvořák P. Continuous beer formation using polyvinyl alcohol entrapped yeast. 28th Congress of the European Brewery Convention, BudapestHungary, May, 12–172001
- Huige N. J., Sanchez G. W., Leidig A. R.. Process for Preparing a Nonalcoholic (less than 0.5 volume percent alcohol) Malt Beverage. US Patent 4,970,082, Nov. 13, 1990
- Back W., Lommi H., Swinkels W. J., Viljava T. T.. (1993) Process for the Production of Non-Alcoholic Beer and Device for Effecting this Process. Euro Patent 601,362 A1, June 15, 1994
- Kaclíková E., Lachovicz T. M., Gbelská Y., Šubík J. Fumaric acid overproduction in yeast mutants deficient in fumarase. FEMS Microbiol. Lett. 1992; 91: 101–106
- Mockovčiaková D., Janitorová V., Zigová M., Kaclíková E., Zagulski M., Šubík J. The ogd1 and kgd1 mutants lacking 2-oxoglutarate dehydrogenase activity in yeast are allelic and can be differentiated by the cloned amber supressor. Curr. Genet. 1993; 24: 377–381
- Navrátil M., Dömény Z., Šturdík E., Šmogrovičová D., Gemeiner P. Production of non-alcoholic beer using free and immobilized cells of Saccharomyces cerevisiae deficient in tricarboxylic acid cycle. Biotechnol. Appl. Biochem. 2002; 35: 133–140
- Navrátil M., Šturdík E., Gemeiner P. Batch and continuous mead production with pectate immobilized, ethanol-tolerant yeast. Biotechnol. Lett. 2000; 23: 977–982