Abstract
Insulin was encapsulated in calcium alginate beads coated with chitosan. Its release from alginate-chitosan and alginate-chitosan-glutaraldehyde beads was studied in artificial gastric (pH 1.2) and intestinal (pH 7.5) fluids. By comparing the release amounts, the ionic interaction between alginate-chitosan matrix with the medium pH's, intestinal fluid was found to be the better. The degradation of released insulin was also searched, even after 6 h incubation, the beads remained stable and the undegraded insulin seemed to be sufficient for the physiological conditions. Consequently, it can be said that the system can be offered for oral delivery of the therapeutic peptide drug insulin.
INTRODUCTION
Diabetes mellitus is a generalized disorder of glucose metabolism that is generally attributed to an insufficiency of insulin. The common treatment for diabetic patients is periodic insulin injection. However this approach is a poor approximation of normal physiological insulin secretion. Therefore, there is a need for self-regulated delivery systems having the capability of adapting the rate of insulin release in response to changes in glucose concentration in order to keep the blood glucose levels within the normal range.Citation[1-2]
Several research groups have been trying to develop insulin delivery systems.Citation[3-4] The efficacy of oral insulin delivery systems for insulin, however is strongly limited by a rapid degradation of this therapeutic peptide by intestinal proteases and a low absorbtion rate. Attempts to overcome the so-called enzymatic barrier include the use of encapsulationCitation[5-6] to provide protection for incorporated insulin towards an enzymatic attack and the development of delivery systems targeting the colon, where the enzymatic activity is comparatively low.Citation[[7]]
After encapsulation, the stability of biological molecules is generally enhanced, toxicity is reduced and they can be released in a controlled way. Alginates, a naturally occuring copolymer of glucuronic acid and manuronic acid, are widely used in biomedical applications and are capable of being processed under mild conditions.Citation[[8]] However calcium alginate beads are very porous and present a low retention capacity of encapsulated molecules. To limit the loss on encapsulated material, the microcapsules are sometimes coated with a polycationic polymer that forms a membrane at the bead surface.Citation[9-11] Microcapsules of calcium alginate coated with polycation have been widely investigated for applications like immunoprotective containers in cell transplantation, enzyme immobilization and drug release systems.Citation[12-14] The immobilization of cells, enzymes and also drugs in calcium alginate polycation microcapsules is a simple and well known techniques.Citation[[5]]
Chitosan is a biocompatible, biodegradable, non-toxic and easily bioabsorbable polycationic polysaccharide derived from the natural polymer chitin.Citation[[15]] Moreover chitosan has antiacid and antiulcer activities that prevent or weaken drug irritation in the stomach. Also chitosan matrix formulation appear to float and gradually swell in acid medium. All these interesting properties of chitosan made this polymer an ideal material for controlled drug release formulations.Citation[16-17] Chitosan membranes have been previously applied to alginate beads formed by droplet extrusion, which are based on strong ionic interactions between the carboxyl residues of the alginate and the amino terminals of the chitosan occur to form a polyelectrolyte complex.Citation[18-20] This complex does not dissolve in the presence of Ca +2 chelators or antigelling cations and thus can be used to stabilize the gel and reduce porosity of the alginate beads.Citation[[21]]
In this present study, the encapsulation of a low molecular weight peptide drug: insulin (5.8 kDa) in calcium alginate beads coated with chitosan is reported. The release depending on the medium pH (artificial gastric and intestinal fluid) was compared. In order to explore chitosan-coated alginate capsules as an oral controlled release system for insulin, the effect of the proteases (pepsin and trypsin), which are mainly responsible for the gastric and intestinal degradation of insulin, was also investigated.
MATERIALS AND METHODS
Alginate was supplied by Fluka Chemie AG (Buchs, Switzerland) and chitosan provided by Marine Chemicals (India). Insulin, trypsin and pepsin was from BDH Ltd. (Poole, UK). All other chemicals and reagents were of HPLC or analytical grade.
Insulin Encapsulation in Alginate-Chitosan Beads
Sodium alginate solution was extruded dropwise, through a flat-end needle using an injector into a gently stirred crosslinking solution. Insulin containing alginate-chitosan beads were prepared by dripping 2% (w/v) sodium alginate with 2%(w/v) insulin into a crosslinking solution composed of 0.1 M CaCl 2, 0.5% (w/v) chitosan and 1% (v/v) acetic acid. The beads were left in the crosslinking solution overnight and then washed several times with distilled water containing 5 mM CaCl 2. Half portion of the beads were then soaked in 0.02 M acetate buffer (pH 5.0) containing 5 mM CaCl 2 and 0.25% (w/v) glutaraldehyde and gently stirred for 60 min. The beads were then washed with distilled water containing 5 mM CaCl 2 to remove the excess of glutaraldehyde. Both preparations (with or without glutaraldehyde) were stored at 4°C for release studies.
Release during bead formation was determined by measuring the concentration of non-capsulated material remaining in the solution used for preparation. The percentage of material released during bead formation and of total release are related to the initial amount of material. The particle size of the beads was determined with the help of a magnifying glass. This was roughly checked by suspending the beads in water contained in a 10 ml of graduated cylinder and measuring the increase in volume.
Release Experiments
In vitro release experiments of insulin were performed with alginate-chitosan and alginate-chitosan-glutaraldehyde beads containing insulin. The beads were placed in glass vials each containing artificial gastric fluid [without pepsin, 2.0 g/L NaCl and 7.0 ml/L concentrated HCl in deionized water (US Pharmacopeia Convention, 1994)] and artificial intestinal fluid [50 mM Tris–HCl buffered saline (TBS) pH 7.5]. The vials were placed into a shaking incubator at 37°C and 100 rpm. At different time intervals, the absorbances were read at 215 nm and 225 nm. The insulin concentration was detected as μg/ml using the 144 A 215–A 225 formula. Insulin release was calculated as follows: where M t is the amount of insulin at time t and M in is the amount of insulin in the beads at time t=0.
Insulin Release Towards Enzymatic Degradation
250 mg of the each set of the beads were placed in 50 mM TBS, pH 7.5 containing Trypsin (10 units/ml) artificial intestinal fluid and artificial gastric fluid with pepsin (5 units/ml). The samples were incubated for 6 h at 37°C, 100 rpm. At 2 h intervals aliquots of 75 μl were withdrawn and diluted with the same volume of 1% trifluoroacetic acid (TFA) used as stop solution in order to terminate the enzymatic reaction. In order to determine the remaining undegraded insulin, 100 μl was directly injected for HPLC analysis (JLC 6000, JASCO Corp.). The HPLC analysis has been carried out according to Michaele et al.Citation[[22]] by some modifications. Insulin and/or degradation products were seperated on Li ChroCART®, Hibar Merck C18 Column 125×4 at 40°C. Gradient elution was performed as follows: flow rate: 0.7 ml/min, 0–17 min, linear gradient 91%A/9%B to 39%A/61%B (eluent A: 0.1% trifluoroacetic acid in water; eluent B: acetonitrile). Insulin and/or degradation products were detected by absorbance at 220 nm. The amount of insulin was quantified from the integrated peak area and calculated by intercorporation from an according standart curve of insulin. Cumulative corrections were made for the previously removed samples in experiments were performed by using 0.5 mg/ml insulin instead of the beads as control.
RESULTS AND DISCUSSION
The encapsulation efficiency for insulin which was entrapped in chitosan-coated alginates was approximately 97% for alginate-chitosan and 95% for alginate-chitosan-glutaraldahyde beads. Although both forms of the beads are uniform and thick, the beads with glutaraldehyde were much more stable in the shaker flask test as evidenced by the breakage rate of the various beads. This may be due to the crosslinking effect of glutaraldehyde during and after gelation that stabilizes them despite the calcium and chitosan detection.
The average diameter for the beads was found to be 1.06 mm; they were relatively uniformly spherical in shape.
In Vitro Release Studies of Insulin
Release profiles of insulin from alginate-chitosan and alginate-chitosan-glutaraldehyde beads were evaluated in two various media and are shown in and , respectively.
Figure 1. Release profile of insulin from chitosan-alginate beads. Beads were incubated in 20 ml release medium (•); artificial gastric fluid, pH 1.2, (◯); artificial intestinal fluid, pH 7.5 on a shaker (100 rpm) at 37°C.
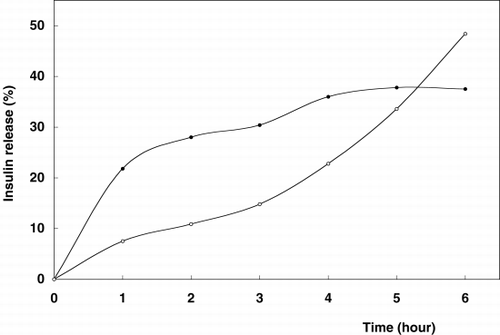
Figure 2. Release profile of insulin from chitosan-alginate-glutaraldehyde beads. Beads were incubated in 20 ml release medium (•); artificial gastric fluid, pH 1.2, (◯); artificial intestinal fluid, pH 7.5 on a shaker (100 rpm) at 37°C.
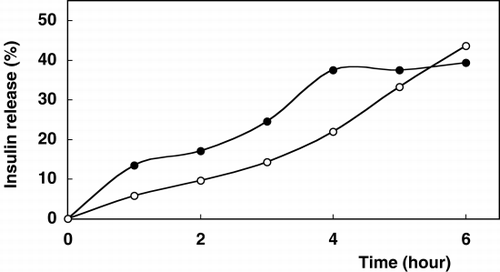
The rates of insulin release are differentiated depending on the medium pH. However, glutaraldehyde had effect in terms of slowing down the release of insulin through the gel core matrix. shows that the glutaraldehyde treatment moderately reduced the diffusion of insulin through the bead membrane. Insulin as a low molecular weight peptide drug was kept in chitosan-coated alginate matrix for the limitation of membrane diffusion. Chitosan membranes are microporousCitation[[23]] and with the pore size increasing with pH levels above 6 due to electrostatic repultion as is easily seen in , especially in the case of intestinal fluid for the sixth hour. This encapsulant-chitosan interaction may be desirable to control release rate and improve the intestinal drugs bioavailability.Citation[[24]] The pH dependent release of some peptides from chitosan-coated microspheres has been analysed. The influence of chemical conformation, chemical composition and the ionic interaction between alginate and chitosan at different pHs were also reported.Citation[[6]], Citation[[25]]
In gastric fluid (pH 1.2) the release profiles of insulin for both type of preparation were characterized by an important initial burst effect (≅58% for alginate-chitosan beads and 34.3% for alginate-chitosan-glutaraldehyde beads) followed by a continuous release phase (release time for insulin: 6 hours). In the case of intestinal fluid, initial release burst effects decreased to ≅17.3% and 17.2%, respectively. As compared to pH 1.2, better release amounts for insulin containing beads were obtained in the TBS (pH 7.5) solution. These phenomenae could be attributed to the removal of the cross-linker bivalent cation calcium from the alginate microspheres by monovalent cations such as sodium contained in TBS. These ion exchangers could cause the erosion of the microspheres and therefore the release of more insulin.
Consequently, after 6 hours total amount of insulin release was observed better under intestinal conditions for both alginate-chitosan and alginate-chitosan glutaraldehyde beads as 48.4% and 43.6%, respectively. In the case of gastric fluid, these values reach up to 37.5% and 39.4%, respectively.
In Vitro Evaluation of the Insulin Release Towards Enzymatic Degradation
Insulin containing alginate-chitosan and alginate-chitosan glutaraldehyde preparations were tested for insulin digestion after release. Concentrations in the test model were adjusted to physiological conditions. Incubation of free insulin in gastric (with pepsin) and intestinal (with trypsin) fluids showed 43.7% and 82% enzymatic degradation, respectively, after 6 hours.
In the case of encapsulated beads depending on the controlled release, undegraded insulin levels were found to be decrasing during 6 hour observation for all mediums as shown in Tables and .
Table 1. In Vitro Insulin Release Towards Enzymatic Degradation Under Gastric Conditions (Artificial Gastric Fluid; pH 1.2 Containing Pepsin at 37°C, 100 rpm)
Table 2. In Vitro Insulin Release Towards Enzymatic Degradation Under Intestinal Conditions (Artificial Intestinal Fluid; TBS pH 7.5 Containing Trypsin at 37°C, 100 rpm)
However, it is thought that remaining insulin amounts still seemed to be sufficientCitation[[26]] at the end of 6 hour incubation.
In conclusion, this study has demonstrated that alginate-chitosan and alginate-chitosan-glutaraldehyde beads may be used for diabetes therapy. Therefore, the beads appear technologically, a promising delivery system for insulin. But, further in vivo studies for the release experiments besides glucose level tests are needed to confirm these observations.
REFERENCES
- Langer R. Drug delivery and targetting. Nature 1998; 392: 5–10
- Kost J., Langer R. Responsive polymer systems for controlled delivery of therapeutics. Trends Biotechnol. 1992; 10: 127–131
- Klumb L. A., Harbett T. A. Design of insulin delivery devices based on glucose sensitive membranes. J. Controlled Release 1992; 18: 59–80
- Ishihara K., Matsui K. Glucose-responsive insulin release from polymer capsule. J. Polym. Sci., Polym. Lett. Ed. 1986; 24: 413–417
- Hari P. R., Chandy T., Sharma C. P. Chitosan/calcium alginate microcapsules for intestinal delivery of nitrofurantoin. J. Microencapsulation 1996; 13: 319–329
- Huguet M. L., Dellacherie E. Calcium alginate beads coated with chitosan: effect of the structure of encapsulated materials on their release. Process Biochem. 1996; 31(8)745–751
- Rubinstein A., Tirosh B., Baluom M., Nassar T., David A., Kabir F., Radai R., Friedman M. The rationale for peptide drugs delivery to the colon and the potential of polymeric carriers as effective tools. J. Controlled Release 1997; 46: 59–73
- Mattiason B. Immobilized Cells and Organnelles, B. Mattiason. CRC, Boca Raton, Florida, USA 1983; Vol. I: 3–25
- Ribeiro A. T., Neufeld R. J., Arnaud P., Chaumeil J. C. Microencapsulation of lipophilic drugs in chitosan-coated alginate microspheres. Int. J. Pharm. 1999; 187: 115–123
- Albarghouthi M., Fara D. A., Saleem M., El-Thaker T., Matalkba K., Badwan A. Immobilization of antibodies on alginate chitosan-beads. Int. J. Pharm. 2000; 206: 23–34
- Gaserod O., Sannes A., Skjak-Braek G. Microcapsules of alginate-chitosan. II. A study of capsule stability and permeability. Biomaterials 1999; 20: 773–783
- Lim F., Sun A. M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980; 210: 908–910
- Soon-Shiong P., Feldman E., Nelson R., Heintz R., Yao Z., Zhang T., Merideth N., Skjak-Braek G., Espeuile T., Smidsrod O., Sandford P. Long term reversal of diabetes by the injection of immunopretected islets. Proc. Natl. Acad. Sci. 1993; 90: 5843–5847
- Pommersheim R., Schrezenmeir J., Vogth W. Immobilization of enzymes by multilayer microencapsules. Macromol. Chem. Phys. 1994; 195: 1557–1567
- Chandra R., Rustgi R. Biodegradable polymers. Prog. Polym. Sci. 1998; 23: 1273–1335
- Grodzinski J. J. Biomedical Application of Functional Polymers. React. Funct. Polym. 1999; 39: 99–138
- Okhamaje A. O., Amsden B., Chu N., Coosen M. F. Modulation of protein release from chitosan-alginate microcapsules using the pH-sensitive polymer hydroxypropyl methylcellulose acetate succinate. J. Microencapsulation 1996; 13: 497–508
- Huguet M. L., Groboillot A., Neufeld R. J., Poncelet D., Dellacherie E. Haemoglobin encapsulation in chitosan/calcium alginate beads. J. Appl. Polym. Sci. 1994; 51: 1427–1432
- Huguet M. L., Neufeld R. J., Dellacherie E. Calcium-alginate beads coated with polycationic polymers: Comparison of chitosan and DEAE-dextran. Process Biochem. 1996; 31(4)347–353
- Takahashi T., Takayama K., Machida Y., Nagai T. Characteristics of polyion complexes of chitosan with sodium alginate and sodium polyacrylate. Int. J. Pharm. 1990; 61: 35–41
- Semidsrot O., Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990; 8: 71–78
- Marschitz M. K., Bernkop-Schnürch A. Oral peptide delivery: polymer-inhibitor conjugates protecting insulin from enzymatic degradation in vitro. Biomaterials 2000; 21: 1499–1507
- Okhamage A. O., Coosen M. F. Control of Membrane Permeability. Fundamentals of Animal Cell Encapsulation and Immobilization, M. F. Coosen. CRC Press, Boca Raton, Florida 1993
- Kristmundsdottir T., Ingvarsdottir K., Saemundsdottir G. Chitosan matrix tablets: the influence of excipients on drug release. Drug Dev. Ind. Pharm. 1995; 21: 1591–1598
- Majeti N. V., Kumar R. Nano and microcapsules of controlled drug delivery devices. J. Pharm. Pharmaceut. Sci. 2000; 3(2)234–258
- Kazmierczak S. C. Clinical Chemistry. Theory Analysis Correlation 3rd Ed., L. A. Kaplan, A. J. Pesce, S. C. Kazmierczak. Moshby Year Book Inc. 1996; 637–638