Abstract
Background: The safety of the hemoglobin based oxygen carrier diaspirin crosslinked hemoglobin (DCLHb) has been reported only in the low (50–200 mg/kg) dose range[Przybelski, R.J.; Daily, E.K.; Kisicki, J.C.; Mattia-Goldberg, C.; Bounds, M.J.; Colburn, W.A. Phase I study of the safety and pharmacologic effects of diaspirin crosslinked hemoglobin solution. Crit. Care Med. 1996, 24 (12), 1993–2000, Bloomfield, E.; Rady, M.; Popovich, M.; Esfandiari, S.; Bedocs, N. The use of diaspirin crosslinked hemoglobin (DCLHb™) in post-surgical critically ill patients. 1996, 95, (3A), A220.]. We conducted a randomized prospective open-label trial of DCLHb and packed red blood cells (PRBCs) in high-blood loss surgical patients to show the effect of 750 ml DCLHb (approximately 1000 mg/kg) on selected indices of organ function. Method: After institutional approval, 24 patients scheduled to undergo elective orthopedic or abdominal surgery, were randomized to receive either PRBCs or 10% DCLHb within 12 hours after the start of surgery. Patients with renal insufficiency, abnormal liver function, severe coronary artery disease (CAD) and ASA physical status≥IV were excluded. The anesthetic technique was left to the judgment of the anesthesiologist. Autologous pre-donation and intraoperative blood conservation techniques were utilized as appropriate. The indications for blood transfusion were individualized on disease state, stage of surgery, and plasma Hb concentration. Laboratory studies were obtained preoperatively and up to 28 days postoperatively. Patients were observed daily for development of jaundice, hematuria, nausea, vomiting, gastrointestinal discomfort, cardiac, respiratory, and infectious complications. Organ effects were assessed with urinalysis, creatinine clearance, electrocardiogram (ECG), and a panel of blood and serum laboratory tests. Results: The dose of DCLHb administered ranged from 680–1500 mg/kg (mean=999 mg/kg). Estimated blood loss was 27±13 ml/kg and 31±15 ml/kg in the control and DCLHb groups, respectively. Fewer PRBCs (1.9±1.2 vs. 3.4±2.4 units, P=0.06) were transfused to DCLHb patients on the operative day although this difference was no longer apparent later on. In the DCLHb group, 4/12 patients avoided any allogeneic PRBC transfusion vs. none in the control group (P=0.09). Systolic, diastolic and mean blood pressure increased moderately after DCLHb for a period of 24–30 hours. There were no occurrences of cardiac ischemia, myocardial infarction, stroke, or pulmonary edema, by clinical or laboratory parameters up to the 28th postoperative day (POD). Seven of 12 (58%) DCLHb patients had yellow skin discoloration vs. none in the PRBC group (P<0.01). Two of four non-urologic surgery patients developed asymptomatic postoperative hemoglobinuria after DCLHb. Creatinine clearance was unchanged postoperatively. Because of hemoglobin interference, bilirubin, γ-glutamyl transferase (GGT), and amylase could not be measured reliably on POD1; on POD2, amylase was transiently elevated to 3 times ULN along with mild elevations of bilirubin, transaminases and BUN. Mean total creatine phoshokinase (CPK) peaked at 8 times the upper limit of normal (ULN) in the DCLHb group, compared with less than twice ULN for controls. Three DCLHb patients had prolonged ileus. Two of these patients had postoperative hyperamylasemia, one of whom developed mild pancreatitis. DCLHb did not affect white blood cell count or coagulation tests. Conclusion: Administration of approximately 1000 mg/kg DCLHb was associated with transient arterial hypertension, gastrointestinal side effects, laboratory abnormalities, yellow skin discoloration, and hemoglobinuria. These observations point to opportunities for improvement in future synthetic hemoglobin design.
INTRODUCTION
While the safety of the U.S. blood supply continues to improve, significant risks and limitations of blood transfusion as well as a patient preference for blood avoidance remain. In the past decade, purified hemoglobin based oxygen carriers (HBOCs) have been readied for clinical testing. The therapeutic potential of HBOCs includes transfusion for perioperative blood loss, trauma resuscitation, hemodilution, augmented oxygen delivery to ischemic tissues or to radiosensitive tumors, treatment of hypotension in sepsis and transfusion in difficult cross-match situations.Citation[[3]] The availability of effective HBOCs may also be potentially life saving in areas of the world where blood banking resources are scarce.
Diaspirin crosslinked hemoglobin (DCLHb, Baxter Healthcare Corporation, Round Lake, Illinois) is a tetrameric hemoglobin molecule derived from human red blood cells whose alpha chains are chemically linked via a fumarate bond. It is primarily eliminated through metabolism in the liver, the reticuloendothelial systemCitation[[4]] and the kidneys. It exhibits saturation kinetics, with a dose dependent plasma half-life. As an HBOC, it supports tissue oxygenation during experimental hemorrhagic shockCitation[5-6] and improves blood flow under various conditions of global and regional ischemia.Citation[7-14] While the manufacturer has discontinued the DCLHb program, other cross-linked hemoglobin species are still being actively investigated for human use.Citation[[15]], Citation[[15a]]
Despite their potential clinical benefits, HBOCs have been associated with a number of side effects. Observations in human volunteersCitation[[1]] and critically ill patientsCitation[[2]] indicate that 25–100 mg/kg DCLHb caused a dose-dependent increase in blood pressure (BP) consistent with pre-clinical observations.Citation[16-18] On average, a maximum increase in mean arterial pressure (MAP) of approximately 30 mmHg was observed at the highest dose tested (100 mg/kg), without associated morbidity or complications.Citation[[19]] Gastrointestinal side effects such as bloating and flatulence also occurred most frequently at the 100-mg/kg dose.Citation[[1]], Citation[[19]] The effective use of molecular hemoglobin during intraoperative hemorrhage necessitates higher doses than were previously studied in humans. In rats, increasing doses (133, 400 and 1200 mg/kg) produced dose-related increases in BP, peripheral vascular resistance and cardiac output, although a ceiling effect was evident at 400 mg/kg.Citation[[20]] It is conceivable that the human cardiovascular and other side effects of DCLHb are different at higher than lower doses.
We therefore sought to evaluate the safety of DCLHb infusion at a dose of approximately 1000 mg/kg. We hypothesized that this dose results in no more serious complications or laboratory changes than would be expected from transfusion of an equivalent or greater amount of red blood cells. Accordingly, we conducted a randomized prospective open trial of DCLHb and PRBCs in patients expected to require more than three units of blood for their surgical procedure.
METHODS
This study was performed under an Investigational New Drug exemption filed with the U.S. Food and Drug Administration. After institutional approval and written informed consent, 24 patients were randomized to receive either DCLHb or PRBCs in an unblinded fashion. Randomization envelopes were opened at the time of the clinically identified need for transfusion. The indications for blood transfusion were individualized based on comorbid disease-state, the stage of the surgical procedure, and the blood hemoglobin concentration. In general, however, patients with known or suspected coronary artery disease (CAD) were transfused below a hemoglobin level of 10 g/dl while those without significant CAD were transfused when hemoglobin level decreased to less than 8 g/dl. In addition, transfusion was indicated when intraoperative blood loss was major and ongoing, or if further bleeding was expected shortly after the time of the transfusion decision.
The study transfusion window was 12 hours starting from the beginning of the surgical procedure. During the 12-hour period after the start of surgery, patients received either a unit of PRBC or 250 ml 10% DCLHb up to 3 units or 750 ml, respectively. Thereafter, PRBCs and other blood products were administered as needed. Only surgical procedures involving the peritoneal cavity, hip replacement, and bilateral knee replacement were included. We excluded patients who had preoperative kidney (serum creatinine>1.7 mg/dl), and liver function test abnormalities (at least 2 tests exceeding the upper limits of normal by 50%). Likewise, patients with a history of prior myocardial infarction without subsequent coronary artery bypass or angioplasty, or patients with ASA physical status IV were excluded.
The anesthetic technique was left to the judgment of the anesthesiologist. Autologous blood pre-donation and intraoperative blood conservation techniques were utilized as appropriate. Immediately prior to each transfusion, one of three investigators (AS, JET, JFO) completed a transfusion indication questionnaire detailing the indication for transfusion. DCLHb was administered using a blood infusion set (SafeLine™ ADDitIV® Primary IV Set, McGaw, Irvine, CA) with a filter (Fenwal® 20 Micron Pediatric Transfusion Filter, Baxter Healthcare Corporation, Deerfield, IL) interposed between the DCLHb container and the infusion set. Both PRBCs and DCLHb were warmed with standard blood warming equipment and transfused as rapidly as clinically needed. DCLHb was shipped frozen from the manufacturer, stored at −20°C in the hospital pharmacy; a minimum of three units was always kept thawed in anticipation of transfusion. In the thawed state, DCLHb was stored at<8°C and discarded after 21 days.
Vital signs and hemoglobin oxygen saturation by pulse oximeter (SpO2) were recorded at 15 minute intervals after each transfusion and at 30 minute intervals after the final transfusion or the third unit of either DCLHb or PRBCs, whichever came first. Laboratory studies were obtained 2, 6, 12, 24, and 48 hours after third unit transfusion and on postoperative days 3, 4, 7, 14, and 28. Intraoperatively, patients were monitored with continuous pulse oximetry, direct arterial pressure, 5-lead ECG and automated real-time ST segment analysis.
All patients were observed daily for development of jaundice, hematuria, nausea, vomiting,Footnote21 gastrointestinal discomfort, cardiac, respiratory, and infectious complications. Jaundice was defined by clinical observers as either a yellow discoloration of the sclerae and/or a generalized yellow skin color. The effect of DCLHb on circulatory, cardiac, renal, hepatobiliary, and hematologic function was assessed by means of a battery of tests. Observers were not blinded to administration of DCLHb because of the procedural difficulty in cloaking the infusion of hemoglobin and the appearance of hemoglobinuria. The severity of adverse events was graded according to a toxicity scale based on the WHO scale.Citation[[21]] Adverse events were described as mild, moderate, severe or life threatening, and were reported as either unrelated, unlikely to be related, probably related or almost certainly related to DCLHb administration.
The control and study groups were descriptively compared on baseline clinical data including age, weight, gender, history of CAD and pulmonary disease, type of surgery and blood loss. Renal and hepatobiliary function tests as well as hematologic and coagulation tests were summarized by group, using mean value, the number of patients exceeding the upper limit of normal (ULN), and the number exceeding three times the ULN. Serum amylase values reported by the laboratory as “< 25 U/L” were assigned a value of 12.5 for statistical analysis. The control and study groups were compared on the proportion with specific complications and on other categorical outcomes (e.g., the proportion with values beyond the ULN) with the Chi-square or Fisher's exact tests. The groups were compared on continuous outcomes (e.g., maximum increase in BP within one hour of start of the DCLHb infusion) with either a t-test or a Wilcoxon rank-sum test where appropriate. For all hypotheses, an overall significance level of 0.05 was used.
RESULTS
A total of 31 patients were enrolled in the study. Of these, 24 required transfusion and therefore were randomized to receive either DCLHb or PRBCs. The dose of DCLHb actually administered (750 ml) ranged from 658 mg/kg to 1500 mg/kg, with a mean of 999±276 mg/kg (SD). The control and study groups appeared to be similar with respect to weight, age, gender, hypertension, (see ) or transfusion indication. Despite random assignment, however, controls had a higher incidence of known cardiovascular disease. The principal transfusion indications listed by the investigators were large blood loss, falling hemoglobin, and falling BP. At no time was it necessary to transfuse to treat myocardial ischemia by EKG.
Table 1. Clinical and Transfusion Data (Mean±SD)
shows transfusion data. All except one patient had 750 ml DCLHb transfused within three hours after first requiring transfusion. Significantly more transfusion events (units of PRBC or 250 ml aliquots DCLHb) occurred intraoperatively in the study group (2.8±0.9 vs. 1.9±1.1 in the control group; P=0.01). In addition, fewer units of PRBCs were transfused to DCLHb patients on the operative day, although this difference was no longer apparent later on (see ). In the DCLHb group, 4/12 patients avoided allogeneic PRBC transfusion vs. none in the control group (P=0.09). A median total of 1427 (range 645–2280) ml PRBC was transfused in the DCLHb group compared to 1388 (705–4080) ml PRBC in the control group.
Table 2. Units PRBC Infused
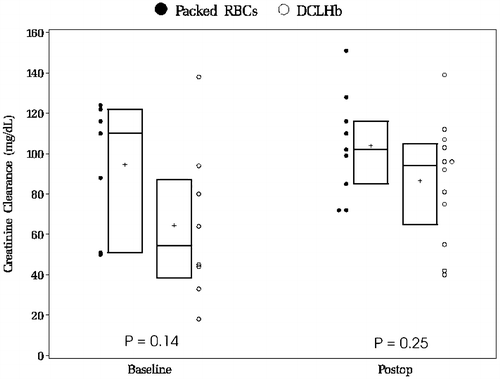
summarizes laboratory data indicative of renal and hepatobiliary function. shows hematologic and coagulation tests. We made no Bonferroni adjustment for multiple comparisons to the significance criterion to be more conservative in our search for potential adverse effects. illustrates the behavior of creatinine clearance after DCLHb or blood transfusion. Ten patients (5 DCLHb and 5 control) were excluded from the calculation because of procedural difficulties and poor patient compliance in preoperative urine collection.
Table 3. Laboratory Tests Indicative of Renal and Hepatobiliary Function (Mean, [SD])Footnote#
Table 4. Hematological and Coagulation Data (Mean, [SD])
Figure 1. Creatinine clearance before and after transfusion displayed with boxplots and raw data points. Boxplot detail: top and bottom are 75th and 25th percentiles, horizontal line in middle is median, ‘+’ is mean.
Seven of 12 patients who received DCLHb (58%) showed evidence of jaundice on physical examination. In contrast, none of the patients receiving PRBCs were observed to have this skin or scleral appearance (P=0.005). On POD 1, bilirubin, GGT, and amylase could not be measured reliably in the majority of patients because of DCLHb interference with spectrophotometrically obtained tests. In the few patients where data points were available, bilirubin was mildly elevated (see ). Likewise, mean AST was only mildly elevated beyond the upper limit of normal (ULN) on POD 1 and 2, compared to control (see A). Although LDH was elevated after DCLHb in hours 1–4 (see B), isoenzyme analysis showed the origin of the elevation to be non-cardiac (i.e., specifically LDH5 was elevated, consistent with the observations of Przybelski et al.Citation[[1]] Total serum creatine kinase was elevated without a concomitant elevation in the isoenzyme creatine kinase myocardial band (CK–MB). Consistent with previous reports,Citation[[22]] antibodies to DCLHb were not detected in any of the samples.
Figure 2. Individual 28-day laboratory profile for each patient in the PRBC and DCLHb groups (A) AST, (B) LDH, (C) amylase. On POD1 data points for amylase are severely reduced because of hemoglobin interference with the assay method. The bold lines represent median values. ULN=upper limit of normal range. Median for DCLHb higher than for PRBC in hours: a) 1–3; b) 1–4; and c) 1–2 (P<0.05 Wilcoxon rank sum tests).
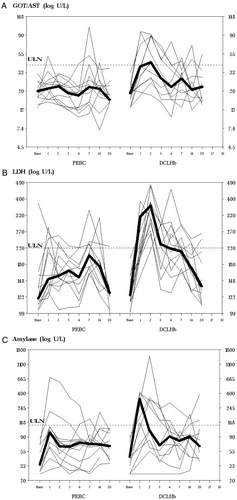
Serum amylase was elevated in 7 of 12 control patients and in 11 of 12 DCLHb patients (see C). Five of 12 DCLHb patients had serum amylase > 3 times ULN compared to 1/12 controls (P=0.08). Mean amylase was elevated to about 3 times ULN on POD2 and returned to normal by POD4 (see c). The incidence of persistent nausea and vomiting during the first 24 hours (least severe on toxicity scale) was 1/12 or 8.3% in each group. Three DCLHb patients who underwent radical cystectomy and prostatectomy with retroperitoneal lymph node dissection had ileus of longer than expected duration. In two of these patients, the ileus was deemed to have prolonged hospital stay. Two of the three patients with ileus had elevations in postoperative serum amylase. Peak amylase concentrations were 351 and 1451 U/L, observed on POD2. The latter patient, who had documented preoperative cholelithiasis, developed mild pancreatitis (by CT scan and clinical course) from which he recovered fully within 4 days.
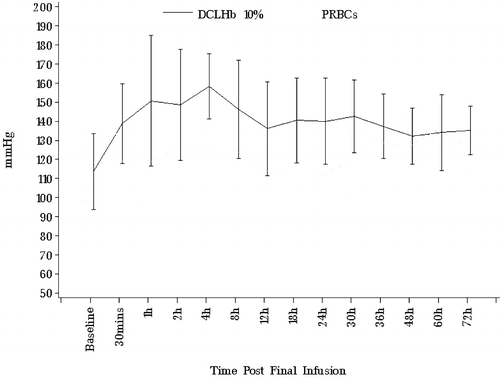
Figure 3. Mean systolic BP (±SD) during the first 72 hours after transfusion of DCLHb or PRBC. Median for DCLHb higher than for PRBC in hours: a) 1–30; b) 1–24; and c) 1–30. (P<0.05 Wilcoxon rank sum tests).
Transient gross hematuria was present in all patients who had urologic and gynecologic surgery. However, red discoloration of the urine was also observed in 2 of 4 patients who received DCLHb and who did not have surgery involving the urologic system. One patient underwent bilateral knee arthroplasty and developed hemoglobinuria at the end of the third unit of DCLHb administration. Hemoglobinuria persisted for 1-hour post-DCLHb administration. Aside from adequate hydration, no specific treatment was necessary and the hemoglobinuria resolved spontaneously. Without affecting renal function. Another DCLHb patient developed hemoglobinuria immediately postoperatively after transabdominal thoracic spinal fusion. It resolved spontaneously within 4 hours without specific therapy. Renal function, as assessed by serum creatinine and creatinine clearance remained normal. DCLHb did not affect serum albumin, while blood cell count, platelet count, or coagulation tests.
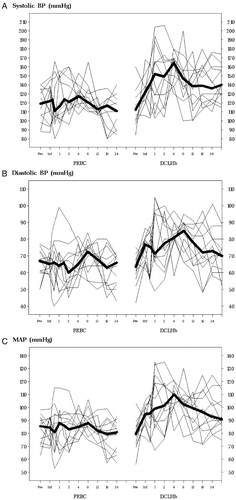
Administration of DCLHb resulted in a sustained increase in BP (see ) of approximately 24–30 hours duration. The median Systolic Blood Pressure (BP), Diastolic Blood Pressure (DBP) and Mean Blood Pressure (MBP) were significantly higher in the DCLHb group during hours 1 through 30 (P<0.05, Wilcoxon rank sum tests). DCLHb patients also had higher mean maximum BP increases within one hour of transfusion start (). All DCLHb patients developed BP >150/90 at least once within thirty hours of the start of the first transfusion (see A and B), regardless of whether they had a pre-existing history of hypertension. Visual inspection of the postoperative blood pressure profiles of DCLHb patients failed to show unusual hypertension in DCLHb patients with a hypertensive history. Three patients had blood pressures > 190/110 mmHg postoperatively, two of whom had a history of pre-existing hypertension. During the first 72-hours after randomization, 7/12 DCLHb patients received antihypertensive medication vs. 2/12 controls (ns). More DCLHb patients (9/12) received furosemide than did controls (3/12). Three of 12 DCLHb patients received medications to increase BP compared with 5/12 controls (ns). Postoperative EKG changes were nonspecific and equally prevalent in control and DCLHb groups. No patient in either group suffered a clinically identifiable cardiac ischemic episode (by symptoms or EKG), a myocardial infarction (by EKG or cardiac enzymes) or pulmonary edema (by symptoms or chest X-ray) during their hospitalization or up to 28 days postoperatively. There were no neurological complications including stroke, transient ischemic neurologic deficit, or unexplained neurologic deficits in either group.
Table 5. Maximum Blood Pressure Increase (mmHg, mean±SD [Median]) within 1 Hour After First Transfusion
Figure 4. Individual BP profiles during the first 24 hours after transfusion: (A) systolic, (B) diastolic, and (C) MAP. The bold lines represent median values. BP=blood pressure; MAP=mean arterial pressure. Median for DCLHb higher than for PRBC in hours: a) 1–30; b) 1–24; and c) 1–30. (P<0.05 Wilcoxon rank sum tests).
DISCUSSION
In this investigation of 750 ml 10% DCLHb as an alternative to PRBC for substantial surgical bleeding, an average dose of approximately 1000 mg/kg DCLHb was associated with transient elevations in serum LDH, AST, total bilirubin, CK, BUN, amylase, a high incidence of yellow skin discoloration and asymptomatic hemoglobinuria. Despite a mild to moderate 24–30 hour elevation in BP after DCLHb, no episodes of myocardial ischemia, myocardial infarction or pulmonary edema were evident.
DCLHb produces a predictable, rapid and sustained rise in MAP, in animal models. The pressure response is dose dependent only in the low dose range (60–125 mg/kg in rats) and pharmacologically reversible. MAP peaked at approximately 130 mmHg and DCLHb's hypertensive response appears to reach a “ceiling” at about 400 mg/kg in rats.Citation[[20]], Citation[[23]] In animals, the duration of MAP increase is also dose-related, ranging from less than an hour at very low doses (35–62 mg/kg), to about 3 hours at doses up to 500 mg/kg and 6 hours at the highest doses (maximum 4000 mg/kg). In human volunteers, 100 mg/kg DCLHb raised median systolic BP maximally by no more than 10 mmHg and diastolic BP by no more than about 15 mmHg, the effect lasting about 3–5 hours.Citation[[1]] However, in patients with chronic renal failure, BP elevations after 100 mg/kg DCLHb persisted beyond 6 hours.Citation[[19]] In the present study, 1000 mg/kg DCLHb raised systolic BP by about 30 mmHg and MAP by about 20 mmHg on average (see A, B and C). At this dose the hypertensive effect in humans appeared to persist for 24–30 hours after administration. Because BP elevations observed with 1000 mg/kg DCLHb approximated prior reported data our results support the existence of a ceiling effect with regard to DCLHb's hypertensive action in humans. They also suggest dose dependence with respect to the duration of hypertension.
In our study, certain factors may have blunted DCLHb's hypertensive tendencies. First, the hypotensive action of surgical hemorrhageCitation[[24]] may have limited the hypertensive response to DCLHb, as did volume depletion during hemodialysis.Citation[[19]] Furthermore, most DCLHb was transfused during general anesthesia, which included potent inhaled anesthetics and barbiturates. Halothane and propofol, but not isoflurane have been shown to decrease the hypertensive action of DCLHb on pulmonary vein rings.Citation[[25]] Although most patients who had general anesthesia received isoflurane as part of their anesthetic regimen, the influence of other anesthetic agents may still have contributed to blunting DCLHb-related hypertension because anesthetic concentration was adjusted at the anesthesiologist's discretion.
Although ongoing surgical blood loss may temporarily mask DCLHb's hypertensive effects, three DCLHb patients in this study had hypertension (BP > 190/110 mmHg) sufficiently severe to require expeditious treatment. Furthermore, all DCLHb patients (vs. only 2/3 of controls) developed mild hypertension postoperatively and significant blood pressure increases were evident even within the first our of DCLHb administration. It is not possible to conclude from our data whether patients with pre-existing hypertension are at greater risk of developing perioperative hypertension. Judging by the extent and duration of hypertension described, DCLHb's hypertensive effects were preserved despite the use inhalation anesthetics and substantial ongoing surgical blood loss.
We observed no grossly adverse renal effects of DCLHb. Although urine collection was likely incomplete in the preoperative period, it was reasonably accurate in the postoperative period. We observed normal postoperative creatinine clearance, unchanged serum creatinine and occasional hemoglobinuria, which required no specific therapy. These results concur with other clinical and pre-clinical studies.Citation[[15]], Citation[[15a]], Citation[26-28] Our observations reinforce the notion that cross-linked hemoglobin does not affect renal function, despite its relatively small molecular size and the occurrence of hemoglobinuria.
The incidence of gastrointestinal side effects was higher in DCLHb patients than PRBC controls. In particular, 58% developed “jaundice”, defined as a clinically apparent yellow hue of skin and sclerae. Hepatic enzymes were not sufficiently elevated to suggest hepatic dysfunction. Skin discoloration probably resulted from mild hyperbilirubinemia due to the increased pigment load and possibly from a tissue “staining” action of DCLHb. Although the incidence of hyperamylasemia was, strictly speaking, statistically not different in DCLHb and PRBC patients, the incidence of severe (>ULN) hyperamylasemia approached statistical significance and inspection of the data is highly suggestive of an association. It should be noted that other synthetic hemoglobins with a molecular structure similar to that of DCLHb also have been associated with hyperamylasemia.Citation[29-31] Mild pancreatitis occurred only in one DCLHb patient, who also had pre-existing cholelithiasis. Of some concern is the occurrence of prolonged ileus in the DCLHb control group. Ileus can be an expected complication of major abdominal surgery, which our patients certainly had. Pancreatitis likewise can occur in the perioperative period after major abdominal surgery with a reported frequency of between 0.8 and 13%.Citation[32-34] Because of the small number of patients affected, the relationship between DCLHb, ileus, hyperamylasemia and pancreatitis cannot be clarified in the context of our study.
The gastrointestinal side effects of DCLHb may be related to its ability to interfere with nitric oxide production and signaling,Citation[[35]] thus possibly affecting gastrointestinal and biliary motility. Rat in vivo studies showed that 400 mg/kg DCLHb increased intestinal and portal system blood flow,Citation[[26]], Citation[[36]] while leaving mesenteric and pancreatic blood flow unaffected. Unless the human gastrointestinal circulation responds differently to DCLHb, the observed side effects are therefore probably not the result of ischemia. Studies are needed to define further the extent of, mechanism for, and potential for prophylaxis against gastrointestinal dysfunction attributable to hemoglobin oxygen carriers.
One of the concerns driving this investigation was the effect of higher doses of DCLHb on the cardiovascular system, particularly in the setting of rapid surgical hemorrhage, which may transiently reduce organ blood flow. While DCLHb is known to increase total peripheral vascular resistance, it augments coronary blood flow at the same time.Citation[[26]] Its effect on cardiac index is more controversial, with some studies reporting a slight decrease,Citation[[37]] and others no change.Citation[[27]] We observed no clinically detectable events of myocardial ischemia or infarction. It should be appreciated, however, that the incidence of documented CAD was lower in our DCLHb patients and our study was not rigorously designed to detect all myocardial ischemia throughout the perioperative period.
The lack of major ischemic side effects on major organ systems of our patients may be explained by the ability of cross-linked hemoglobin to counteract tissue hypoperfusion with added blood oxygen carrying capacity and perfusion pressure. In spontaneously hypertensive rats subjected to middle cerebral artery occlusion, hemodilution with DCLHb (to hematocrits of 30, 16 or 9%) resulted in a significantly reduced extent of brain injury and cerebral edema.Citation[[38]] The most effective reductions in ischemic injury occurred in those animals in which the inherent hypertensive response to DCLHb was not inhibited. In contrast to typical catecholamine effects, the pressor response of DCLHb can be associated with an increase in perfusion (as indicated by organ flow measurements) in both top-load and hemorrhagic, hypovolemic animal models.Citation[[6]], Citation[[15]], Citation[[15a]] DCLHb, compared with non-oxygen containing crystalloid or colloid solutions resulted in substantially better survival from experimental hemorrhagic shock in both ratsCitation[[6]] and swine.Citation[[15]], Citation[[15a]] This salutary effect may be related to DCLHb's effect on tissue perfusion and peripheral oxygenation, measured at the tissue level.Citation[[7]]
The current study should be interpreted with the realization that its results are subject to a number of substantial limitations. DCLHb's effect on many direct functional aspects of organ performance, such as cardiac index, oxygen extraction or hepatic excretion was not studied directly. Likewise, important physiological parameters such as pulmonary artery pressure, systemic lactate concentration and acid base status were not measured. Moreover, at least within the first 24–36 hours of administration, DCLHb interferes with the photospectrometric determination of a variety of laboratory tests. At this institution, such interference was particularly noticeable for bilirubin, amylase, albumin, GGT and alkaline phosphatase. Therefore, no definitive conclusions about the early values of these test results can be reached based on the present study (see ). Interference with laboratory testing constitutes an important limitation for the potential clinical use of artificial hemoglobin species. Our study protocol ensured patient safety with increased patient surveillance and alternative diagnostic pathways. Because the study design did not include investigator blinding, it is conceivable that the incidence of subjectively observed side effects was influenced by observer bias. Given possible “hypervigilant” behavior on the part of observers looking for previously known side effects in the DCLHb group, the incidence of “jaundice”, for example, may be overstated.
Our results indicate that administration of DCLHb at doses up to 1000 mg/kg during rapid surgical bleeding is associated with systematic hypertension, transient mild to moderate gastrointestinal side effects, enzyme elevations, skin discoloration and hemoglobinuria. DCLHb under these conditions was not associated with cardiac, hepatic, hematologic or renal complications. However, it's side effects profile illustrates the need for improvement in future synthetic hemoglobin design.
ACKNOWLEDGMENTS
The authors thank the entire study DCLHb support team for their outstanding efforts. We are indebted to Dr. Richard Van Lente, Dr. Ronald Domen, Carolyn Bratush, and Dori Odar from the Departments of Clinical Pathology, Primary Laboratory Medicine, and Blood Bank and Transfusion Medicine, for their dedicated and insightful handling of laboratory testing. We would like to thank Mr. Ed Jones, Investigational Pharmacist, for his contribution, as well as Drs. David Goldfarb, Tracy Hull, Elroy Kursh, Kenneth Angermeier, Craig Zippe, Stuart Flechner, Peter Brooks, Jerome Belinson, David Miller, Iain Kalfas, Timothy Sullivan, Alexander Kennedy, Elisa Ross, for allowing their patients to participate. We wish to acknowledge the dedication and competency of Nita Marie Bedocs, Peggy Imler, and Jody Kolat, Study Coordinators.
Notes
1Nausea or vomiting requiring repeated therapeutic intervention.
REFERENCES
- Przybelski R. J., Daily E. K., Kisicki J. C., Mattia-Goldberg C., Bounds M. J., Colburn W. A. Phase I study of the safety and pharmacologic effects of diaspirin crosslinked hemoglobin solution. Crit. Care Med. 1996; 24(12)1993–2000
- Bloomfield E., Rady M., Popovich M., Esfandiari S., Bedocs N. The use of diaspirin crosslinked hemoglobin (DCLHb™) in post-surgical critically ill patients. Anesthesiology 1996; 95(3A)A220
- Dietz N. M., Joyner M. J., Warner M. A. Blood substitutes: fluids, drugs or miracle solutions?. Anesth. Analg. 1996; 82: 390–405
- Keipert P. E., Gomez C. L., Gonzales A., MacDonald V. W., Hess J. R., Winslow R. M. Diaspirin crosslinked hemoglobin: Tissue distribution and long-term excretion after exchange transfusion. J. Lab. Clin. Med. 1994; 123(5)701–711
- Cohn S. M., Farrell T. J. Diaspirin cross-linked hemoglobin of hemorrhage: Comparison of a blood substitute with hypertonic saline and isotonic saline. J. Trauma 1995; 39(2)210–216
- Schultz S. C., Powell C. C., Bernard E., Malcolm D. Diaspirin crosslinked hemoglobin (DCLHb™) attenuates bacterial translocation in rats. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1994; 22: A260
- Powell C., Schultz S. C., Burris D. G., Drucker W. R., Malcolm D. S. Subcutaneous oxygen tension: A useful adjunct in assessment of perfusion status. Crit. Care Med. 1995; 23(5)867–873
- Schultz S. C., Hamilton I. A., Jr., Malcolm D. S. Use of base deficit to compare resuscitation with Lactated Ringers solution, haemaccel, whole blood and diaspirin crosslinked hemoglobin following hemorrhage in rats. J. Trauma 1993; 35: 619–626
- Powell C., Schultz S. C., Burris D. G., Brucker W. R., Malcolm D. S. Resuscitation with diaspirin crosslinked hemoglobin (DCLHb™) restores subcutaneous oxygen tension as well as blood in rats. Surg. Forum 1993; 44: 40–42
- Powell C. C., Schultz S. C., Malcolm D. S. Diaspirin crosslinked hemoglobin (DCLHb): More effective than lactated Ringer's solution in restoring central venous oxygen saturation after hemorrhagic shock in rats. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1996; 24(3)197–200
- Frankel H. L., Nguyen H. B., Shea-Donohue T., Aiton L., Ratigan J., Malcolm D. Diaspirin cross-linked hemoglobin is efficacious in gut resuscitation as measured by a GI tract optode. J. Trauma 1996; 40(2)231–240
- McKenzie J. E., Cost E. A., Scandling D. M., Savage R. W. Effects of diaspirin crosslinked hemoglobin (DCLHb™) on cardiac function and CG in the swine. Biomater., Artif. Cells, Immobilization Biotechnol. 1992; 20(2–4)683–687
- Cole D. J., Schell R. M., Drummond J. C., Reynolds L. Focal cerebral ischemia in rats. Effect of hypervolemic hemodilution with diaspirin crosslinked hemoglobin versus albumin on brain injury and edema. Anesthesiology 1993; 78(2)335–342
- Cole D. J., Schell R. M., Drummond J. C. Diaspirin crosslinked hemoglobin (DCLHb): Effect of hemodilution during focal cerebral ischemia in rats. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1994; 22(3)813–818
- Viele M. K., Weiskopf R. B., Fisher D. Recombinant human hemoglobin dose not affect renal function in humans: Analysis of safety and pharmacokinetics. Anesthesiology 1997; 86(4)848–858
- Toussant M., Latger-Cannard V., Caron A., Lecompte T., Stoltz J. F., Vigneron C., Menu P. Effects of three Hb-based oxygen-carrying solutions on neutrophil activation in vitro: Quantitative measurement of the expression of adherence receptors. Can. J. Anaesth. 2001 Apr.; 48(4 Suppl.)S41–S48
- Przybelski R. J., Daily E. K., Birnbaum M. The Pressor Effect of Hemoglobin—Good or Bad?. Advances in Blood Substitutes: Business Opportunities and Medical Challenges, R. M. Winslow, K. D. Vandegriff. Birkhaeuser, Boston 1977; Vol. 3: 71–90
- Przybelski R. J., Daily E. K. The Pressor/Perfusion Effect of Diaspirin Crosslinked Hemoglobin (DCLHb™). Yearbook of Intensive Care and Emergency Medicine, J. L. Vincent. Springer-Verlag, Berlin 1994; 253–263
- Farmer M. C., Przybelski R. J., McKenzie J. E., Burhop K. E. Preclinical Data and Clinical Trials with Diaspirin Crosslinked Hemoglobin. Artificial Red Cells, E. Tsuchida. John Wiley & Sons Ltd., New York 1995; 177–185
- Swan S. K., Halstenson C. E., Collins A. J., Colburn W. A., Blue J., Przybelski R. J. Pharmacologic profile of diaspirin crosslinked hemoglobin in hemodialysis patients. Am. J. Kidney Dis. 1995; 26(6)918–923
- Barve A., Sen A. P., Saxena P. R., Gulati A. Dose response effect of diaspirin crosslinked hemoglobin (DCLHb) on systemic hemodynamics and regional blood circulation in rats. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1997; 25: 75–84
- Przybelski R. J., Daily E. K., Stern K. N., Mattia-Goldberg C. A graded scale for the assessment of safety of blood substitutes. Transfusion 1997; 37: 749–751
- Patel M. J., Webb E. J., Shelbourn T. E., Mattia-Goldberg C., George A. J., Zhang F., Moore E. G., Nelson D. J. Absence of immunogenicity of diaspirin cross-linked hemoglobin in humans. Blood 1998; 91(2)1–8
- Malcolm D. S., Hamilton I. N., Schultz S. C., et al. Characterization of hemodynamic response to intravenous diaspirin crosslinked hemoglobin solution in rats. Artif Cells, Blood Substitues, Immobilization Biotechnol. 1994; 22: 91–107
- Malcolm D., Kissinger D., Garrioch M. Diaspirin crosslinked hemoglobin solution as a resuscitative fluid following severe hemorrhage in the rat. Biomater., Artif. Cells, Immmobilization Biotechnol. 1992; 20: 495–497
- Jing M., Ledvina M. A., Bina S., Hart J. L., Muldoon S. M. Effects of halogenated and non-halogenated anesthetics on diaspirin crosslinked hemoglobin induced contractions of porcine pulmonary veins. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1995; 23(4)487–494
- Sharma A. C., Gulati A. Effect of diaspirin crosslinked hemoglobin and norepinephrine on systematic hemodynamics and regional circulation in rats. J. Lab. Clin. Med. 1994; 123(2)299–308
- Rhea G., Bodenham A., Mallick A., Przybelski R., Daily E. Initial evaluation of diaspirin crosslinked hemoglobin (DCLHb) as a vasopressor in critically ill patients. Crit. Care Med. 1997; 25(9)1480–1488
- Baron J. F., Berridge J., Brichant J. F., Demeyere R., Lamy M., Larbuisson R., Lehot J. J., Parsloe M., Sinclair C. J., Vandermeesch E., Group Hemassist Cardiac Surgery Trial Collaborative. The use of diaspirin crosslinked hemoglobin (DCLHb) as an alternative to blood transfusion in cardiac surgery patients following cardiopulmonary bypass: a pivotal efficacy trial. Anesthesiology 1997; 87(3A)A217
- Leone B. J., Chuey C., Gleason D., Steele S. M., et al. Can recombinant human hemoglobin make ANH more effective? An initial feasibility and safety study. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1996; 24: A379
- Lessen R., Williams M., Seltzer J., Lessin J., et al. A safety study of recombinant human hemoglobin for intraoperative transfusion study. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1996; 24: A380
- Gould S. A., Moore E. E., Moore F. A., Haenel J. B., et al. Clinical utility of human polymerized hemoglobin as a blood substitute after acute trauma and urgent surgery. J. Trauma, 43(2)325–332
- Jurkovich G. J., Carrico C. J. Pancreatic trauma. Surg. Clin. North Am. 1990; 70: 575–593
- Miyagawa S., Makuuchi M., Kawasaki S., Kakazu T. Changes serum amylase level following hepatic resection in chronic liver disease. Surgery 1994; 129: 634–638
- Akagi Y., Yamashita Y., Kurohiji T., et al. Investigation of hyperamylasemia after hepatectomy. J. Jpn. Soc. Clin. Surg. 1991; 52: 314–318
- Sharma A. C., Sinh G., Gulati A. Role of NO mechanism in cardiovascular effects of diaspirin crosslinked hemoglobin in anesthetized rats. Am. J. Physiol. 1995; 269(4 Pt. 2)H1379–H1388
- Sharma A. C., Rebello S., Gulati A. Regional circulatory and systemic hemodynamic effects of diaspirin crosslinked hemoglobin in the rat. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1994; 22(3)593–602
- Lamy M. 1997, Personal communication
- Cole D. J., Schell R. M., Drummond J. C., Przybelski R. J., Marcantonio S. Focal cerebral ischemia in rats: effect of hemodilution with α-α crosslinked hemoglobin on brain injury and edema. Can. J. Neurol. Sci. 1993; 20: 30–36