Abstract
Cellulose adsorbent with amphiphilic ligands for adsorption of low density lipoprotein (LDL) was prepared by the following procedures: Cellulose beads were reacted with cholesterol N-(6-isocyanatohexyl) carbamate in the presence of pyridine in DMSO at 80°C in order to introduce the hydrophilic moiety, it was then reacted with chlorosulfonic acid in dimethyl formamide, which introduced the sulfonic group. Adsorption capacity of adsorbent was studied, which showed the removal of LDL, TC, TG to be 0.857 mg/ml, 1.317 mg/ml, 1.002 mg/ml respectively without significantly affecting total protein levels in the plasma. Moreover, it has a better selectivity in removing LDL, TC, TG compared with cellulose adsorbent with only hydrophobic or hydrophilic ligand.
INTRODUCTION
Low density lipoprotein (LDL) is widely recognized as one of the major risk factors for developing ischemic heart diseases (IHD).Citation[1-2] Various techniques have been used to control plasma LDL levels to prevent the occurrence and/or progression of IHD and to treat IHD in patients with refractory hyperlipidemia.Citation[[3]] Aggressive removal of LDL cholesterol from hyperlipidemia patients utilizing an extraporeal technique, i.e. hemoperfusion has been proven to be beneficial, preventing and regressing coronary atherosclerosis. Cellulose adsorbents were first used in blood purification because of its good biocompatibility and strength compared to PVC, agar and other materials. Since late 1970s, many researchers have been engaged in developing different kinds of cellulose materials.Citation[4-6] Up to date two types of LDL adsorbents have been reported i.e. hydrophobic and hydrophilic.Citation[7-13] So far, no corresponding observation has been reported for cellulose adsorbents with amphiphilic ligands. In this paper, cellulose adsorbent with amphiphilic ligands was first synthesized and its properties and factors affecting its adsorption capacity were investigated.
MATERIALS AND METHODS
Materials
Cellulose beads were synthesized by our laboratory; Cholesterol (purity>99%) was purchased from TBD (Beijing, P.R.C.); Hexamethylene Diisocyanate(GC) was purchased from Kasei Kogyo Co., Ltd. (Tokyo, Japan); Dimethyl formamide (DMF) (Analytical grade) was purchased from Beijing Chemical Reagents Co., Ltd. (Beijing, P.R.C.); Pyridine (Analytical Grade) was purchased from Tianjin Chemical Reagents Co., Ltd. (Tianjin, P.R.C); Chlorosulfonic acid (Analytical Grade) was purchased from Beijing Fuxing Chemical Reagents Factory (Beijing, P.R.C.). All other reagents are analytical grade. Plasma was taken from several patients aged between 45–65 years old with hypercholesterolemia from Tianjin General Hospital, who were not taking lipid-lowering drugs or drugs altering metabolism of lipid for at least 2 months.
Synthesis of Cellulose Adsorbent with Amphiphilic Ligands
The coupling of cholesterol to cellulose beads was carried out by reacting hexamethylene diisocyanate with the –OH group of cholesterol.Citation[14-15] Briefly, 0.3 mmol of hexamethylene diisocyanate was allowed to react with 20.2 mmol of cholesterol and stirred at 80°C for 24 h in the presence of catalyst (pyridine) in dried tolune; then the solute was distilled at reduced pressure. 600 ml petroleum ether was added to the remaining solution, and stored at −20°C over night.The solution was centrifuged at −4°C, the precipitate was collected and dried in vacuum.
Dried cholesterol N-(isocyanatohexyl) carbamate and cellulose beads were mixed in 200 ml dried DMSO, then 10 ml pyridine was added and stirred at 80°C under an atmosphere of nitrogen for 8 hours. The beads were washed several times with ethanol and dried in vacuum.
Cellulose adsorbent with cholesterol ligand was placed in 100 ml dimethyl formamide solution, then chlorosulfonic acid was dropped into the solution slowly and stirred at 4°C for five hours; the final product was washed several times with absolute methanol and distilled water. The adsorbent was stored at 4°C for further use.
Determination of Cholesterol Ligand and Sulfonic Group Contents
The content of cholesterol ligand and sulfonic group was determined by a Perkin–Elmer-2400C element analytical meter.
Determination of Adsorption Capacity
0.5 ml of cellulose beads were incubated with 2.0 ml serum and stirred for 3 hours at 37°C, respectively; Total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), and total proteins (TP) were determined by using the corresponding commercial test kits purchased from Zhongsheng High Tech Bioengineering Company (Beijing, P.R.C).
RESULTS AND DISCUSSION
Effect of Adsorption Time on Adsorption Capacity
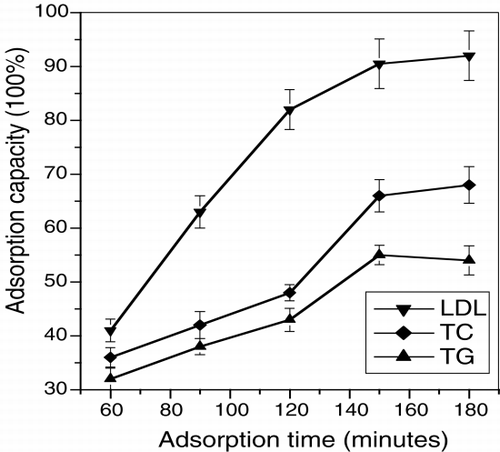
illustrates the amount adsorbed at different adsorption intervals. The adsorption rate increased with the increment of adsorption time at 3 hours, it reached an optimal condition, the adsorption capacity for LDL, TC and TG was 0.857 mg/ml, 1.317 mg/ml and 1.002 mg/ml respectively.
Effect of the Ligand Ratio on Adsorption Capacity
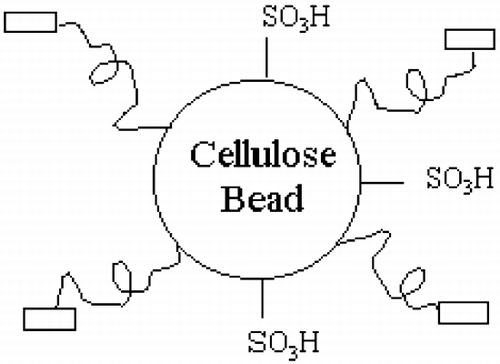
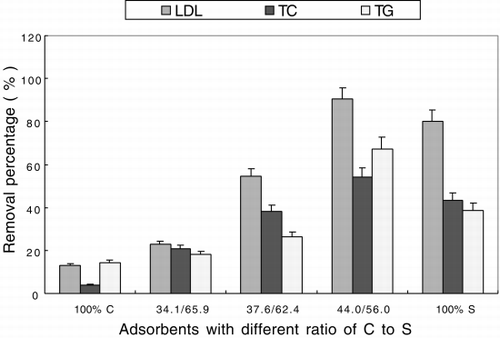
According to the analytical data of cellulose adsorbents listed in , the molar ratio of cholesterol to sulfonic group could be calculated. shows the schematic structure of adsorbent, cellulose adsorbent has not only hydrophobic cholesterol ligands, but also hydrophilic sulfonic groups. The molar ratio of cholesterol to sulfonic group could be adjusted by controlling reaction condition. The adsorption capacities with different ratios of cholesterol ligand to sulfonic group are listed in and shown in . From , it is evident that adsorbent containing only hydrophobic moiety i.e. cholesterol, its adsorption capacity for LDL, TC, TG was less than 10%.This may be because hydrophobic interaction is weak and requires a long spacer for interaction between big molecules.Citation[[17]] The spacer in the present experiment contained only 6 carbon chain. The adsorption capacity of amphiphilic was much higher than the above two types of adsorbents. It increased as the content of sulfonic group increased and reached a maximum at 56.0% with a capacity for LDL, TC and TG of 0.857 mg/ml, 1.317 mg/ml and 1.002 mg/ml respectively. After the maximum value, the adsorption capacity decreased for reasons unknown which may involve hydrophobic and hydrophilic interactions. In addition, amphiphilic cellulose adsorbent could also adsorb HDL (adsorption capacity for HDL ∼20%). Further study is required to reduce the adsorption capacity for HDL.
Table 1. Analytical Data of Cellulose Adsorbent
Table 2. Analytical Data of Cellulose Adsorbents
Effect of the Adsorbent on Plasma Total Protein Adsorption
The non-specific adsorption of the new adsorbent was tested for its adsorption of total plasma protein. Results showed that the total plasma protein adsorption was less than 10%, which indicated that the adsorbent has a good selectivity.
Storage Stability
Wet state cellulose adsorbent was stored at 4°C in refrigerator. The adsorption efficiency of adsorbent at 0, 2, 4, 8, 12 weeks were determined. No significant decrease in adsorption efficiency was observed after 12 weeks of storage at 4°C.
CONCLUSION
Cellulose adsorbent with amphiphilic ligands has many advantages that makes it an ideal adsorbent for removal of LDL, TC, TG in plasma with high efficacy and good selectivity. Preliminary studies shows that it has a promising prospective in future.
ACKNOWLEDGMENTS
The research work was supported by The National Key Project of Fundamental Research and Advances (G1999064707).
REFERENCES
- Kannel W. B., Castelli W. P., Gorden T., McNamara P. M. Serum cholesterol, lipoprotein and the risk of coronary heart disease: The Framingham study. Ann. Intern. Med. 1971; 74: 1
- Lipid Reseach Clinics Program. The lipid research clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. J. Am. Med. Assoc. 1984; 251: 365
- Tani N. Development of selective low-density lipoprotein (LDL) apheresis system: Immobilized polyanion as LDL-specific adsorption for LDL apheresis system. Artif. Organs 1996; 20(8)922–929
- Kojima S., Harada-Shiba M., Toyota Y., Kimura G., et al. Changes in coagulation factors by passage through a dextran sulfate cellulose column during low-density lipoprotein apheresis. Int. J. Artif. Organs 1992; 15(3)185–190
- Sato Y., Agishi T. Low-density lipoprotein adsorption for arteriosclerotic patients. Artif. Organs 1996; 20(4)324–327
- Gohdes M., Mischnick P. Determination of the substitution pattern in the polymer chain of cellulose sulfates. Carbohydr. Res. 1998; 309: 109–115
- Borberg H. H., Gaczkowski A., Hombach V. Treatment of familial hypercholesterolemia by means of a specific immunoadsorption. J. Clin. Apheresis 1988; 4: 59–65
- Stoffel W., Demant T. Selective removal of apolipoprotein B-containing serum lipoprotein from blood plasma. Proc. Natl. Acad. Sci. U. S. A. 1981; 78: 611–615
- Stoffel W., Borberg H., Greeve V. Application of specific extracorporeal removal of low density lipoprotein in familial hypercholesterolemia. Lancet 1981; 78(11)1005–1007
- Stoffel W., Bode C. Selective Removal of Low Density Lipoproteins. Selective Plasma Component Removal, A. A. Pineda. Futura Publ. Co., Mount Kisco, New York 1984; 1–22
- Maaskant N., Bantjes A., Kempen H.J. M. Removal of low density lipoprotein from blood plasma using cross-linked, sulfated polyvinylalcohol. Atherosclerosis 1986; 62: 159–166
- Bosch T. Low-density lipoprotein hemoperfusion using a modified polyacrylate adsorber: in vitro, ex vitro, and first clinical results. Artif. Organs 1996; 20(4)344–345
- Bosch T., Schmidt B., Kleophas W., Gillen C., Otto V., Passlick-Deetjen J., Gurland H. J. LDL hem perfusion—a new procedure for LDL apheresis: First clinical application of an LDL adsorber compatible with human whole blood. Artif. Organs 1997; 20(9)977–982
- Sreenivasan K. Synthesis and evaluation of β-cyclodextrin-2-hydroxyethyl methacrylate copolymer as a novel adsorbent. Polym. Int. 1997; 42: 22–24
- Sreenivasan K. Grafting of β-cyclodextrin-modified 2-hydroxyethyl methacrylate onto polyurethane. J. Appl. Polym. 1996; 60: 2245–2249
- Chen B., Pan J., Tong M., Yu Y., et al. A study of adsorbents for the adsorption of low density lipoprotein (LDL). Ion Exch. Adsorpt. 1993; 9(4)330–334
- Cao N.-N., Chen C.-Z., Yu Y.-T. In vitro study of a novel low density lipoprotein adsorbent. Artif. Cells, Blood Substitutes, and Immobilization Biotechnol. 2002; 30: 53–61