Abstract
The bioconversion of domestic wastewater sludge by immobilized mixed culture of filamentous fungi was investigated in a laboratory. The potential mixed culture of Penicillium corylophilum WWZA1003 and Aspergillus niger SCahmA103 was isolated from its local habitats (wastewater and sludge cake) and optimized on the basis of biodegradability and dewaterability of treated sludge. The observed results in this study showed that the sludge treatment was highly influenced by the effect of immobilized mixed fungi using liquid state bioconversion (LSB) process. The maximum production of dry filter cake (DFC) was enriched with fungal biomass to about 20.05 g/kg containing 23.47 g/kg of soluble protein after 4 days of fungal treatment. The reduction of COD, TSS, turbidity (optical density against distilled water, 660 nm), reducing sugar and protein in supernatant and filtration rate of treated sludge were influenced by the fungal mixed culture as compared to control (uninnoculated). After these processes, 99.4% of TSS, 98.05% of turbidity, 76.2% of soluble protein, 98% of reducing sugar and 92.4% of COD in supernatant of treated sludge were removed. Filtration time was decreased tremendously by the microbial treatment after 2 days of incubation. The effect of fungal strain on pH was also studied and presented. Effective bioconversion was observed after 4 days of fungal treatment.
INTRODUCTION
Increasing volumes of waste sludge combined with limited space availability and progressively tightening environmental standards has promoted the development of new intensive biotechnological processes for treatment of wastewater sludge. Currently available conventional biological wastewater treatment techniques are mostly incapable of complete elimination of organic and toxic substances in stable forms. Alternatively physical treatment does not present real degradation but only a replacement of the treated substances in a concentrated waste volume.Citation[[1]] In Malaysia, Indah Water Konsortium (IWK) produces enormous quantities of waste sludge daily in different wastewater treatment plants. About 3 million cubic meters of sludge is being generated every year and it will be increased to 7 million cubic meters by the year 2020.Citation[[2]] Safe, scientific and environmentally friendly disposal of these huge quantities of generated sludge are the main concerned.
Effective solid-liquid separation persists to be a major problem in various operation units in wastewater treatment.Citation[[3]] Among these, sludge dewatering has been pointed out as one of the most expensive and least understood processes.Citation[[4]] Non soluble and soluble compounds are two kinds of pollutants encountered in wastewater. The treatment of non soluble substances is achieved easily by mechanical separation processes e. g. sedimentation, flocculation, flotation, hydrocycloning and filtration. On the other hand the treatment of soluble compounds is more difficult and so expensive. The conventional solutions used at present are adsorption (only for low concentration), oxidation (this is very expensive for dilute organic compounds) and membrane processes (low cut-off molecular weight is needed for the removal of soluble compounds). The present study proposes a new solution for the biodegradation of soluble and insoluble pollutant of waste sludge in LSB process.Citation[[5]] In this process the filamentous fungi can immobilize on waste particles of sludge and enhance the separation and filtration process by their potential growth. The soluble and insoluble materials in sludge can be assimilated by the microbial treatment. Hence the removal of organic substances can be increased significantly by the immobilized microbial treatment. Therefore the purpose of the present study was to evaluate the potential effect of immobilized mixed fungi on waste sludge for the biodegradation and biological separation using bioconversion process.
MATERIALS AND METHODS
Fungal Strains
A mixed culture of Penicillium corylophilum WWZP1003 (IMI385277) and Aspergillus niger SCahmA103 (IMI385267) obtained from a series of experiments of isolation/identification,Citation[[6]] screening and optimization of mixed culture was used. P. corylophilum and A. niger were isolated from relevant sources (wastewater and sludge cake) and optimized on the basis of potential performance for the treatment of domestic wastewater sludge using bioconversion process. The cultures were maintained on 3.9% w/v of potato dextrose agar (PDA) slants, subcultured once in a month and stored at 4°C.
Wastewater Sludge
The domestic wastewater sludge (0.75–1% w/w of TSS, pH 6.6) was collected from an aeration tank in a sewage treatment plant, Kuala Lumpur, Malaysia. The substrate of 1% w/w TSS of sludge supplemented by co-substrate of 2% w/w of wheat flour (WF, optimized carbon source) was used throughout the study.
Inoculum Preparation
The cultures were grown on PDA in petri dishes at room temperature (30±2°C) for 7 days. Three plates (petri dish) of fungal cultures were then transferred into Erlenmeyer flask (250 ml) containing 100 ml of sterile distilled water. It was shaken in a rotary shaker at 150 rpm for 24 h. The suspended fungal mycelia were filtered through filter paper (Whatman) and the filtrate was used as inoculum after recording its concentration (spores/ml) with Haemocytometer.
Experimental Conditions and Procedures
The optimized cheap carbon source (C) and process parameters were used to evaluate the bioconversion process. The optimized C source was wheat flour and the process parameters such as temperature, initial pH, inoculum size and co-substrate (WF) concentration were 33°C, 5.5, 2% v/w, 2% w/w, respectively. To evaluate the microbial performance for waste sludge treatment, an experiment was carried out in a 500 ml Erlenmeyer flask containing 100 gm of wastewater sludge medium with 2% w/w of wheat flour as co-substrate. The samples were autoclaved at 121°C for 30 min and inoculated with mixed culture of 2% v/w of inoculum (1% v/w of inoculum for each fungus). It was incubated in a rotary shaker at 150 rpm for 6 days (sampling was carried at everyday). The spore suspension of inoculum was used in the experiment. The concentration of mixed inoculum of P. corylophilum and A. niger used were 1.25×104 and 2×104 spores/ml respectively.
Analytical Methods
The dry filter cake was collected by filtering the fermentation broth and dried at 105°C for 24 hrs. The supernatant was used for analysis after centrifuging the fermented broth at 3000 rpm for 30 min. The TS, TSS, and COD were measured following the standard methods.Citation[[7]] The optical density (OD) of supernatant at 660 nm was measured against distilled water in an UV spectrophotometer (UVIKON 933). Protein was determined according to Lowry method.Citation[[8]] Reducing sugar was measured according to the method of DNS.Citation[[9]] The filterability of fermentation broth was determined by measuring the filtration time of every 10 ml of the filtrate to 50 ml of total. The filtration was carried out under a constant vacuum of 300 mmHg. Immobilization/entrapment of fungal mixed culture of A. niger and P. corylophilum in waste sludge were analysed by using an image analysis system consisting of a microscope, a CCD camera, a PC and an image analysis software (Leica Qwin 5001). The mycelial pellets/flocs formed by fungal strains after 2 days of sludge treatment were surface viewed by JEOL JSM-6400 (Japan) which was operated at 15 kV according to the method of Flegler et al.Citation[[10]] The experiment was carried out by completely randomize design (CRD) and results obtained were the average of three replicates.
RESULTS AND DISCUSSION
The potential mixed microfungi of P. corylophilum and A. niger were used for the treatment of wastewater sludge in optimized process conditions. The effect of microbial treatment on bioconversion of sludge was higher compared to control treatment (untreated/uninnoculated) in terms of dry filter cake (DFC) enriched with protein in treated sludge, and removal of TSS, soluble protein, reducing sugar as substrate utilization, optical density (OD) as turbidity measurement and COD in supernatant, pH and increased filterability of treated sludge. The results of this study revealed that the bioconversion was highly influenced by fungal treatment compared to uninnoculated sample.
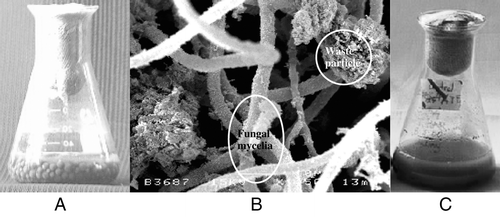
shows that the fungal mixed culture was immobilized in waste particles of sludge (1% w/w of TSS) and its growth was transformed into mycelial pellets after 2 days of treatment. The entrapment of solid particles by potential microbe could increase the settleability and dewaterability of treated sludge. Immobilization of P. chrysosporium has been studied by Alam et al.Citation[[5]] for the bioconversion of domestic wastewater sludge. It was reported that the recovery of coal fines by oil agglomeration technique and biofilm formation was successfully achievedCitation[[11]] by exploiting fungal activities.
Figure 1. The immobilization of fungal mixed culture of P. corylophilum and A. niger in waste particle of sludge (a) the fungal growth formation as pellets after 2 days of treatment; (b) surface viewed of pellets in SEM; (c) control (uninoculated).
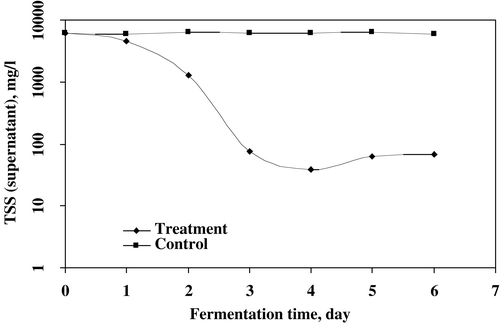
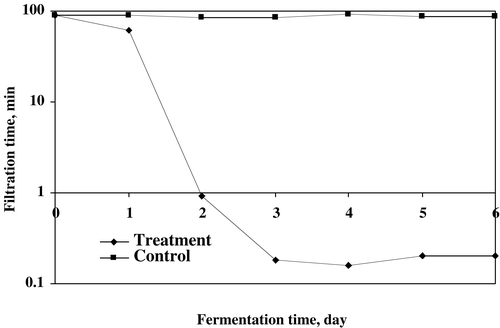
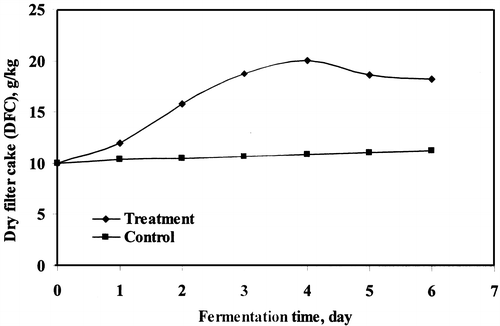
The dry filter cake (DFC) in treated sludge and TSS in supernatant were observed up to 6 days of treatment. The highest increased rate for DFC and decreased rate for TSS (supernatant), both parameters were recorded after 4 days of treatment ( and ). The maximum 20.05 g/kg of DFC enriched with 24.28 g/kg of soluble protein in DFC was achieved in microbial treatment of wastewater sludge. The highest increased percentage of DFC was recorded (100.2%) in 1% w/w of sludge at 4 days of fermentation. The DFC production was slightly decreased after 4 days of treatment. Several workers have studied the production of dry filter cake for different wastes.Citation[[5]], Citation[12-13] The maximum removal of TSS was 99.4% in supernatant at 4 days of treatment and after that it was slower for 5 and 6 days of fermentation. Jin et al.Citation[[14]] has removed 95% of suspended solids in starch processing wastewater using fungal strains.
Figure 2. The increased rate of dry filter cake (DFC) production by microbial treatment of wastewater sludge.
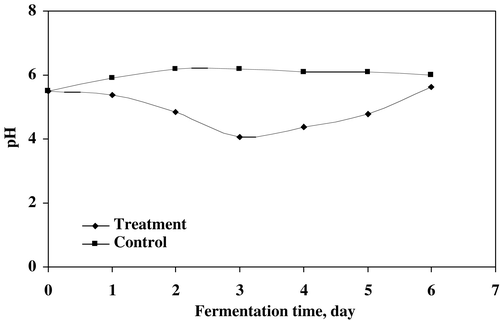
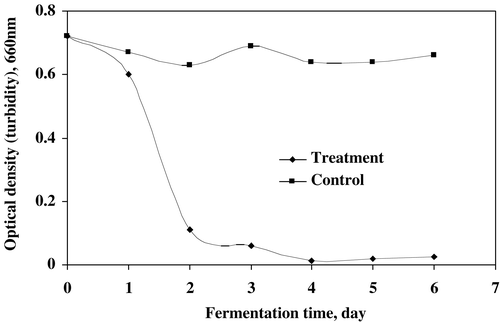
The effect of microbial treatment on the reduction of turbidity in supernatant is shown in . The fungal strains utilized the soluble organic substances resulting in higher reduction of turbidity (OD 0.014, 660 nm) at 4 days of treatment. The decreasing trend was observed for the first four days after that it increased a little up to 6 days of treatment. The value of pH was affected by the fungal growth in sludge treatment as shown in . Initially the value decreased up to 3 days of treatment while it increased (pH 5.63) during the final days of incubation. The fungal strains produced acidic metabolites due to their acid producing characteristics.
Figure 4. The turbidity (optical density against distilled water at 660 nm) in supernatant of treated sludge.
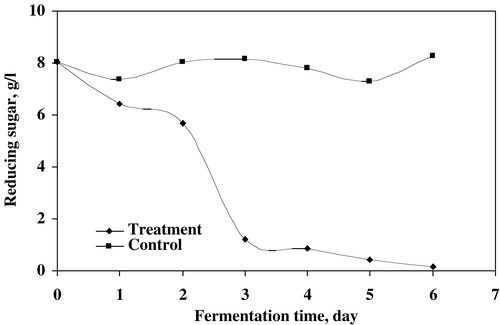
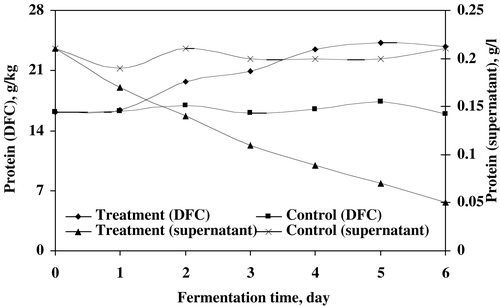
The reducing sugar as substrate utilization by microfungi was measured in treated sludge (). The results indicated that the microbial mixed culture utilized 98% of the substrate in sludge for proper growth and formation. The reduction rate was observed up to the final days of treatment. The protein recorded in DFC and supernatant was shown in . In DFC, the protein was enriched with biomass resulting in increased of 33.5% of protein compared to the control while the protein in supernatant was decreased by fungal treatment.Citation[15-16] This could be due to the utilization the dissolved protein as nutrient and energy source. The maximum reduction of dissolved protein in supernatant observed was 76.2% after treatment. The highest increased and decreased values of protein in DFC and supernatant respectively, were achieved after 6 days of microbial treatment.
Figure 7. The increased and decreased rate of protein in DFC and supernatant of treated sludge, respectively.
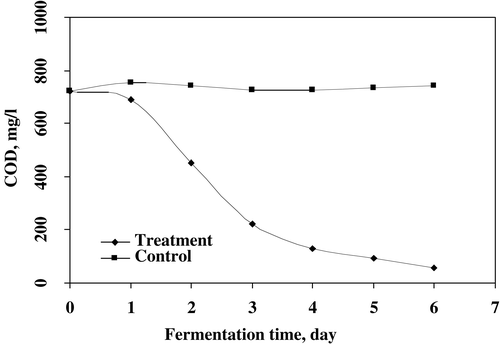
The removal of COD of treated sludge was accelerated by using fungal mixed culture of P. corylophilum and A. niger for 6 days of treatment (). The reduction rate of COD was highly influenced by the immobilized microbial treatment of waste sludge. The results indicated that 94.2% of removal of COD was recorded after 6 days of treatment, which was the highest compared to those 1–5 days and uninnoculated sample (0 days). The increasing rate of removal of COD was studied in different waste sludges such as domestic wastewater sludge, olive oil mill waste, apple distillery waste, kraft bleaching effluents and pulp and paper mill effluent using fungal strains.Citation[[5]], Citation[[12]], Citation[17-20] The effect of fungal treatment on increasing rate of filtration is illustrated in . The faster rate to filter the fermentation broth was recorded by using potential mixed fungal inoculum for first 2 days of treatment. No significant difference was observed after two days of operation. The results showed that the filtration rate of treated sludge were about 550 times faster than that of uninnoculated sample. The fungal cultures immobilized/entrapped the solid particles of sludge and enhanced the separation and filtration process.Citation[[12]], Citation[[21]]
CONCLUSION
The results presented in this study showed that the potential immobilized mixed fungal inoculum had a high microbial and enzymatic effects which led to an increase biomass protein containing DFC, reduction of dissolved protein, reducing sugar, COD, TSS, and turbidity in supernatant and filtration time of treated sludge using the LSB process. The immobilization of fungi in waste particles of sludge enhanced the separation process which helped to increase the filtration rate. It is a new biotechnological approach to considering the economic importance for the treatment of wastewater sludge by enhancing the biodegradability and dewaterability of treated sludge. This study may contribute to better sludge management strategy for the bioconversion of higher solid content of sludge into compost by solid state bioconversion (SSB) process in future research.
ACKNOWLEDGMENTS
The authors would like to convey their heartfelt gratitude to Universiti Putra Malaysia (UPM) for their kind supports and IWK for their research grant and for providing the sludge sample during this study.
REFERENCES
- Scholz W., Fuchs W. Treatment of oil contaminated wastewater in membrane biorector. Water Res. 2000; 34(14)3621–3629
- Kadir M.D. A., Velayutham S. The Management of Municipal Wastewater Sludge in Malaysia. Symp. on Sludge Management, Universiti Technologi Malaysia. Aug., 18–191999
- Lorain O., Thiebaud P., Badorc E., Aurelle Y. Potential of freezing in wastewater treatment: soluble pollutant applications. Water Res. 2001; 35(2)541–547
- Bruus J. H., Nielsen P. H., Keiding K. On the stability of activated sludge flocs with implications to dewatering. Water Res. 1992; 26: 1597–1604
- Alam M. Z., Fakhru'l-Razi A., Molla A. H., Roychoudhury P. K. Treatment of wastewater sludge by liquid state bioconversion process. J. Environ. Sci. Health 2001; A36(7)1237–1243
- Fakhru'l-Razi A., Alam M. Z., Idris A., Abd-Aziz S., Molla A. H. Filamentous fungi in Indah Water Konsortium (IWK) sewage treatment plant for biological treatment of domestic wastewater sludge. J. Environ. Sci. Health 2002; A37(3)309–320
- A.P.H.A. Standard Methods for the Examination of Water and Wastewater17th Ed. American Public Health Association, Washington, DC 1989
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951; 193: 265–275
- Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959; 31: 426–428
- Flegler S. L., Heckman J. W., Klomparens K. L. Scanning and Transmission Electron Microscopy. W. H. Freeman and Company, New York 1993
- Sharma S., Luxamikant, Dastidar M. G., Roychoudhury P. K. Recovery of coal fines from washery and power plant effluents by integrated technique of oil agglomeration and biofilm formation. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1999; 27(5 and 6)387–392
- Friedrich J., Cimerman A., Perdih A. The use of Aspergillus niger for bioconversion of apple distillery waste. Eur. J. Appl. Microbiol. Biotechnol. 1983; 17: 243–247
- Friedrich J., Cimerman A., Perdih A. Comparison of different cellulolytic fungi for bioconversion of apple distillery waste. Appl. Microbiol. Biotechnol. 1986; 24: 432–434
- Jin B., Van Leeuwen J., Yu Q., Patel B. Screening and selection of microfungi for microbial biomass protein production and water reclamation from starch processing wastewater. J. Chem. Technol. Biotechnol. 1999; 74: 106–110
- Friedrich J., Cimerman A., Perdih A. Mixed culture of Aspergillus awamori and Trichoderma reesei for bioconversion of apple distillery waste. Appl. Microbiol. Biotechnol. 1987; 26: 299–303
- Nigam P. Process selection for protein-enrichment: fermentation of the sugar industry by products molasses and sugar beet pulp. Process Biochem. 1994; 29: 337–342
- Hamdi M., Hamed H. B., Ellouz R. Optimisation of olive mill waste-waters by Aspergillus niger. Appl. Microbiol. Technol. 1991; 36: 285–288
- Marwaha S. S., Grover R., Prakash C., Kennedy J. F. Continuous biobleaching of black liquor from the pulp and paper industry using an immobilized cell system. J. Chem. Technol. Biotechnol. 1998; 73: 292–296
- Nagarathnamma R., Bajpai P., Bajpai P. K. Studies on decolorization, degradation, and detoxification of chlorinated lignin compounds in kraft bleaching effluents by Ceriporiopsis subvermispora. Process Biochem. 1999; 34: 939–948
- Robes A., Lucas R., Alvarez de Cienfuegos G., Galvez A. Biomass production and detoxification of wastewaters from the olive oil industry by strains of Penicillium isolated from wastewater disposal ponds. Biores. Technol. 2000; 74: 217–221
- Hamdi M., Ellouz R. Use of Aspergillus niger to improve filtration of olive mill waste-waters. J. Chem. Tech. Biotechnol. 1992; 53: 195–200