Abstract
Type X collagen is principal extracellular matrix (ECM) in natural dermis. To prepare artificial dermis, collagen is traditional, and most superior biomaterial. But beside collagen, the dermis also contains many other ECM. Among them, glycosaminoglycan (GAG) is another important substance. To imitate the natural dermis, and modificate the scaffold materials, two types of scaffolds were prepared: one is traditional type X collagen spongy scaffold, the other is collagen-chondroitin sulfate (CS) spongy scaffold. Collagen was blended with CS, one kind of GAG, and cross-linked by 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC). Dermis fibroblast was isolated from neonate prepuce, and dermis fibroblasts were cultured on the scaffolds. The physical and chemical properties of the scaffolds were tested, including SEM, DSC, H&E staining, immunohistochemical staining and CS content analysis and so on. The results indicated that EDC is an effective and non-cytotoxic cross-link reagent, and attaching CS into collagen scaffold could improve the stability and histocompatibility of scaffold.
INTRODUCTION
For years, the defect of skin is a severe problem for surgeons. Every year, the number of burns add up to about 60,000∼80,000. Although autologous skin graft has been successfully used for deep burns, this kind of treatment inflicts additional injures, and there were not sufficient amounts of autographs for large area skin defect. The surgeons have used cadaver skins and animal skins, but most of implantation cases implied that they can only served as temporary wound dressings, they cannot cover wound permanently and induce skin regeneration. From 1975, because of the development of cell culture technology, the use of growth factor and the find of neutral proteinase Dispase, the artificial skin graft has proceeded expeditiously.Citation[[1]] But surgeon has verified that after the dermatoplasty without dermis, keloid is easily formed in the surface of wound, the ability of anti-infect is very lower and the surface of wound easily forms ulceration. That is said, the artificial dermis is needed under that dermatoplasty.
To resolve this problem, natural collagen was attached with chondroitin sulfate to constitute the scaffold for imitating the natural substitute of skin ECM. Dermal fibroblast was isolated from neogenesis prepuce, and cultured in the scaffold we prepared. By comparing the property of those two scaffolds, it can be concluded that the collagen-CS scaffolds is a kind of biomaterials better than the collagen scaffold in skin tissue engineering. The artificial dermis, which consists of collagen and chondroitin sulfate, has proximal structure with normal dermis and will be more useful in the therapy of skin defect.
MATERIALS AND METHODS
Preparation of Scaffold Materials
Preparation of Collagen Swelling Solution
Collagen was extracted from bovine tendon and was treated by the method of enzyme digestion described in Zhang et al.Citation[2-3] 63.5 ml 0.2 M acetic acid was added in 36.5 g 1.37% collagen swelling solution to get 0.3%(w/v) collagen swelling solution. The solution was homogenized in a high speed stirrer and was defoamed for 10 min in a freeze centrifuge (2000 rpm, 5X).
Preparation of Collagen Scaffold
8 ml collagen swelling solution was poured onto 60 mm culture dishes. And then dried in freezes dryer to form the collagen lattice. The membrane was cross-linked by 0.3% formaldehyde (PH 8.4) for 2 h. The cross-linked membrane was washed in double-distilled water repeatedly. At last the membrane was frozen at −40X 2 h, and lyophilized in frozen dryer again.Citation[4-5]
Preparation of Collagen-Chondroitin Sulfate Scaffold
At room temperature, 50 mg (dry weight) collagen lattice was marinated in 50 mM 2-morpholinoethanesulphonic acid (MES), 20 ml 40% ethanol for 30 min. And then the collagen lattice was cross-linked by immersing in 20 ml 40% ethanol containing 50 mmol/L MES (PH 5.5), 33 mmol/L EDC and 0.2% CS (w/v) for 4 h. After cross-linking, the lattice was washed in 0.1 mol/L Na2HPO4 (PH 9.1), 1.0 mol/L NaCl and double-distilled water respectively, and lyophilized at last.Citation[[6]]
Physical Characterization of Scaffolds
Scanning Electron Microscopy
The specimens of the scaffolds were prepared for scanning electron microscopy (SEM) examination by fixation, dehydration in alcohol, freezes drying and coating with gold. The effect of cross-linking on matrix morphology and porosity was observed by HI-TACHI X-650 SEM.
Differential Scanning Calorimetry
The thermal character of matrices was determined with differential scanning calorimetry by TADSC2910. The range of temperature was 30∼300X at a heating rate of 10X/min in nitrogen atmosphere.
Swelling Properties
Three specimens from each of two materials were weighed by analytical balance (Wd), and then immersed in PBS solution for 2 h at room temperature. Take photo of the two scaffolds. After absorbed the additional water, weighed again (Wh). The swelling degree (Sw) was calculated by the following formula.
Isolation and Culture of Dermis Fibroblasts
Separate and Culture of the Neonatal Dermal Fibroblast
The skin samples were obtained from neonate prepuce. Then the skin was cut into 1×1 cm2 in asepsis, and washed in the D X Hanks solution containing penicillin (100 u/ml) X streptomycin X 100 ug/ml XX amphotericin BX2.5 ug/mlX. The skin spices were incubated in 10 ml dispase (0.25%) at 4X for 1–2 h. After that, the epidermis was removed, the dermal layer was cut to pieces and then added 10 ml tape X collagenase (0.25%) into the solution. After digested 0.5–1 h at 37X, the suspension was collected, filtered by cell filter (×200), centrifuged 1500 rpm for 5 min. The cells were collected, resuspended in culture medium (DMEM) with 10% fetal calf serum, 100 IU/ml penicillin and 100 IU/ml streptomycin. Then the fibroblast cultures were maintained at 37X in air and 5% CO2.Citation[[7]]
Implantation of the Fibroblast
The collagen scaffold was cut into circle of 16 mm in diameter, then putted into 24-well culture plate, and sterilized by X irradiation. After culturing for 3–4 generation, 0.1 mL medium of cells suspension at a density of 1.0×107 cells/ml were implanted into the collagen scaffolds on the 24-cell culture plate which have been immersed in the DMEM solution for 30 min before implantation. After 3 hours, 1 ml DMEM culture solution with 10% fetal calf serum was added to each well. The culture plate were culture at 37X, 5% CO2 the culture solution was changed each other day.Citation[8-9]
Biochemical Analysis
Immunohistochemical Analysis
At each week end, the samples were fixed by 4% paraformaldehyde X dehydrated by graded alcohol X embedded in paraffin X and sliced into section, X the sections were thick 4 umX. The sections were stained with H&E method. And the immunohistochemical demonstration of fibronectin (FN) and type I collagen were performed by standard techniques, Vectastain ABC Kit, and monoclonal rat antibody against human FN and monoclonal rat antibody against human type I collagen were used.
Glycosaminoglycan (GAG) Content
The content of the GAG was measured by methylene blue method. The samples were cut into spices and digested by tape X collagenase X 2.5 mg/mlX at 55X for 24 h. After cooled, the digested solution was mixed. Then 0.1 ml that mixture was added into 2 ml 1,9-dimthyl methylene blue staining solution. The samples were placed at room temperature for 10 min, and their absorption was measured at 535 nm.
RESULTS
Physical Characterization of Scaffolds
Scanning Electron Microscopy
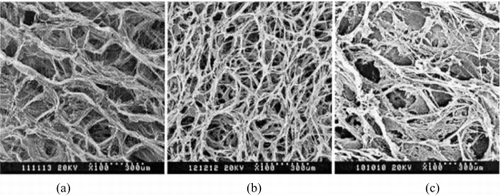
From the SEM photos (), we can see that the scaffolds were evenly porous and three-dimension interconnected fiber structure. The pore size of the collagen scaffold is 50–150 μm and the rate of porosity is 94%. Comparing the cross-linked scaffold with uncross-linked scaffold, we found that the cross-linked scaffold was stable in size, a little toughness, and the porosity was more equilibrated. In compare with collagen scaffold, the pore size of the collagen-CS scaffold was smaller with more conglutination structure. Between the collagen fibers, there is abundant CS.
Differential Scanning Calorimetry
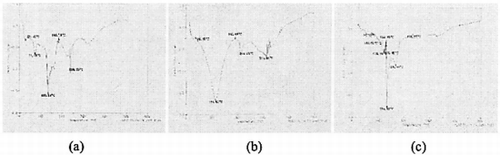
The result of DSC about collagen scaffold and collagen-CS scaffold were showed as . With the DSC analysis of the scaffolds, the thermodynamics of the materials were investigated to show the interaction among the components of the materials.
Swelling Properties
The photos of two scaffolds in PBS were shown in .
Figure 3. (A) The structure of collagen-CS scaffold soaking in PBS solution. (B) The structure of collagen spongy scaffold soaking in PBS solution. (×250).
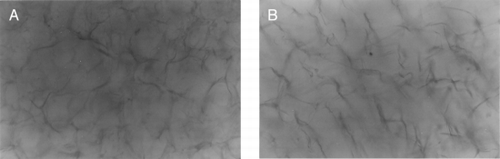
In , the swelling properties of these two scaffolds were compared. The collagen-CS scaffold's swelling degree is higher than the collagen scaffold's.
Table 1. Swelling Properties of Collagen and Collagen-CS ScaffoldsFootnotea
Separation and Culture of the Fibroblast
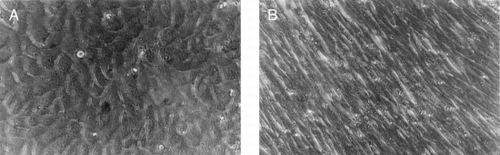
The fibroblasts isolated from dermal with cell activity of more than 90% were implanted into culture flask. After 4 hours, 95% cells adhesive to the wall. The cells were typical spindle-shape. After 3–4 days, the cells generated once. The morphology of the cells has no obviously difference ().
Biochemical Analysis
H&E Staining
The scaffolds without fibroblast appeared very light in hematoxylin with the H&E stains. After implanted one week, the cells on the scaffold surface were dispersed among the scaffold. The cells on the scaffold surface were rounding, oval and fusiform in the scaffold. But the number of the cells is very small. The cells on the collagen scaffold were lying between the fibers of scaffold, and the cells on the collagen-CS have attached to the scaffold (A). At the third week, the number of the cells among the scaffold increased obviously. The extracellular matrix (ECM) secreted by the fibroblast could be seen on the scaffold. The collagen structure is still whole with a little part began to degrade. For these two scaffolds, the degradation of the collagen-CS scaffold is not obviously and has more ECM than the collagen scaffold (B). At the fifth week, the number of the cells has been debased, and these two scaffolds have been degraded. But the ECM secreted by fibroblast has increased very much (C).
Immunohistochemical Analysis
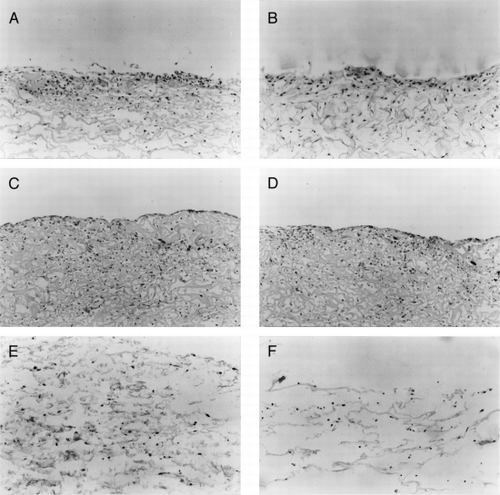
At the first week, the stains of the FN and type I collagen of the two scaffolds were all light. From the second week, the stains were increased. At the end of fourth week, the stains of the two scaffolds have been strong positive. By Comparing the two scaffolds, the collagen-CS scaffold' stains were deeper than collagen scaffold's (, ).
Figure 6. The immunohistological staining of collagen-CS scaffold after fibroblasts cultured for (A) one week, (C) 2 weeks, and (E) 4 weeks. The immunohistological staining of collagen scaffold after fibroblasts cultured for (B) one week, (D) 2 weeks, and (F) 4 weeks. (X250).
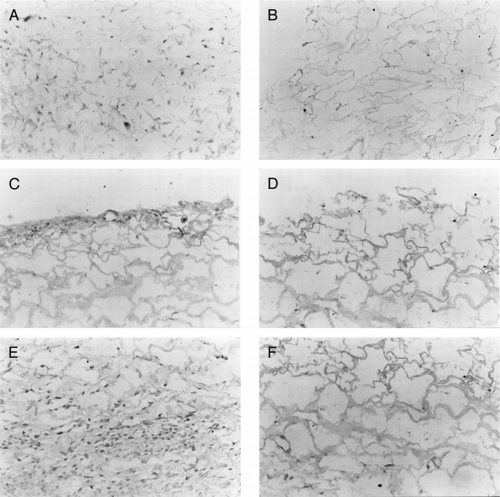
Table 2. The Stains of the FN and Type I Collagen of the Two ScaffoldsFootnotea
Glycosaminoglycan (GAG) Content
After the cells were implanted in the scaffolds, the content of GAG in the scaffolds raised persistently as the has showed. From first week to fourth week, the content of GAG in collagen scaffold has increased about three times from 15±1.3 to 45±1.5 μg/well. And the GAG content in collagen-CS scaffold has increased about five times from 30±1.4 μg/well to 162±1.1 μg/well. In compare to the first week, those two scaffolds' GAG content all increased obviously (P<0.01).
Table 3. The GAG Content in Two Scaffolds μg/well
DISCUSSION
The natural dermis is composed by fibroblast and ECM, such as collagen, proteoglycan, and so on. Fibroblast is a kind of mesenchymal cell. It can secrete ECM, growth factor. ECM is the environment that the fibroblast lives. Being a kind of ECM, collagen is very important in tissue engineering research. It has good histocompatibility, mechanical character and biodegradability. In 1980, Yanna and Burke use collagen sponge to prepare artificial skin.Citation[[4]], Citation[[10]] And now the INTEGRA™ has been used to deep burning. The collagen sponge has good biocompatibility and guided tissue regeneration capability. It can accelerate the wound healing and decrease the scar forming. Although collagen has so many excellence characteristics, it is not the only substance that conform the natural dermis ECM. Beside collagen, GAG is an other important ECM that conform the dermis. To imitate the natural dermis and improve the performance of scaffold; CS, a kind of GAG, was blended into our scaffold. We also ameliorate the technology of cross-linking, using EDC to substitute formaldehyde. And the dermis fibroblasts isolated from neonate prepuce were implanted into the scaffold to manufacture the artificial skin that has bioactivity.Citation[11-13]
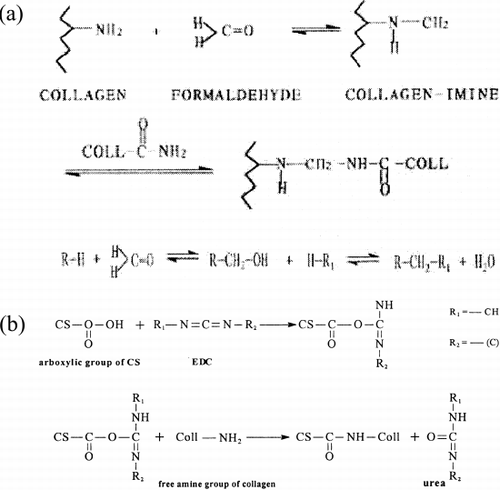
In the studies, the acetic acid was used as swelling solution, because collagen swells easier in it. The acetic acid's volatility is stronger than malonic acid too, and can be easily washed from the scaffold. As the cross-linking agent, EDC was used in constituting collagen-CS scaffold. Though formaldehyde is a very important cross-linking agent to improve the physical and chemical properties (the reaction mechanism is shown in a), it is poisonous to tissue cells. Carboddiimide compounds are used as coupling reagents of the protein by activating the carboxyl group and forming amide with amine. Cross-linked by EDC, the scaffold is not only more stable but also can be blended CS by covalent bond (the reaction mechanism is shown in b). Being as active regent, the final product of EDC is urea and moved away by washing. So it is no poisonous to tissue.Citation[14-15]
Figure 7. (a) Reaction mechanism of formaldehyde crosslinking the collagen. (b) Reaction mechanism of EDC crosslinking the collagen and EDC.
In the photos of SEM (), the scaffold materials have conformed net frame. The aperture size of the scaffolds is 50–150 um, and about 70% pore size is 80–100 um. The aperture of collagen-CS is more uniform than collagen. The pore of collagen-CS is less than collagen. After dipped in PBS, the fabric of the scaffolds hasn't changed obviously, and the structure has maintained well ().
As we known, the damaging of the super-helix structure of the protein secondary structure causes the denaturation of the collagen protein. In a, there was a small absorption peak at 71.15X, that was formed by denaturation of noncollagen in the scaffold. But in b and c the peak at 71.15X has been disappear. That was probably because the heterproteins in collagen have been denatured after cross-linking. In c, there was a stronger absorption peak at 168.44X in the collagen-CS scaffold. That was the peak of CS's phase change. And the collagen-CS scaffold's denature temperature of 134.30X was higher than collagen scaffold's. So it can be concluded that attaching CS in the collagen scaffold could improve the stabilization of the materials.
In the study, we used the method of Hansburgh and Middelkoop: the neogenesis prepuce dermal fibroblast was used. The neogenesis prepuce dermal fibroblast can propagate rapidly in vitro with high activity and secret abundant ECM. The results show that the fibroblast adhered and grows well on the collagen scaffold with and without the CS. And it can secret FN and type I collagen on the collagen matrix, the rate and the content of secretion on collagen-CS scaffold are all higher than the collagen scaffold.Citation[16-17]
The results of the histochemical analysis show that the collagen scaffold is good to the generation of the cells and the forming of the ECM. The blending CS in the scaffold will improve the materials' biocompatibility, and it can increase the strength of the scaffold and decrease the degradation. The results of the GAG content measurement shows that the fibroblast implanted on the collagen scaffold can secrete abundance ECM, and there is more ECM on the collagen-CS scaffold. The content of the GAG is an important substance besides the type I collagen in the ECM. After attaching GAG into the collagen sponge, the strength of the matrix can increase and the degrading rate in vivo will be delayed. The results show the fibroblast adhered and grows more rapidly on the scaffold with CS than that without CS. The secretion of ECM is also higher in collagen-CS scaffold. The reason is maybe that the collagen scaffold combined CS has the proximately content as the normal ECM, so it is suitable for the cells growth, generation and secreting ECM.Citation[[18]]
CONCLUSION
Attaching CS into collagen scaffold can improve the physical performance, boost up the scaffold's strength, and prolong the degradation of scaffold. And these remodifications along with substituting formaldehyde by EDC for cross-linking optimize the scaffold's biological performance. The collagen-CS scaffold cross-linked by EDC has less poisonous to tissue cells and much higher histocompatibility. It can be concluded that the collagen-CS film cross-linked by EDC is more suitable as scaffold for artificial skin.
ACKNOWLEDGMENT
This work is supported by the National Natural Science Foundation of China for Prominent Youth (No. 59625306) and National Emphasis Basis Subject (973 Program) G1999054309-4.
REFERENCES
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocyte: The formation of keratinocyte colonies from single cells. Cell 1975; 6: 331–334
- Zhang Q., Yao K., Liu L., et al. Evaluation of porous collagen membrane in guided tissue regeneration. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1999; 27(3)245–253
- Zhang Q., Liu L., Ren L., et al. Preparation and characterization of collagen-chitosan composites. J. Appl. Polym. Sci. 1997; 64: 2127–2130
- Yannas I. V., Burke J. F. Design of an artificial skin: I. Basic design principles. J. Biomed. Mater. Res. 1980; 14: 65–81
- Shuuichi I., Nobuyuki S., Yoshimitsu K. Preparation of composite cultured skin and its evolution after grating. J. Jan. P.R.S. 1991; 11: 515–531
- Boyce S. T., Christianson D. J., Hansbrough J. F. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J. Biomed. Mater. Res. 1988; 22: 935–957
- Bell E., Bengt I., Charlotte M. Production of a tissue like structure by contraction of collagen lattice by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. U. S. A. 1979; 76(3)1274–1278
- Dermarchez M., Hartman D. J., Regnier M., et al. The role of fibroblasts in dermal vascularization and remodeling of reconstructed human skin after transplantation onto nude mouse. Transplantation 1992; 54(2)317–326
- Black A. F., Berthod F., Lheureux N., et al. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998; 12(13)1331–1340
- Yannas I. V., Burke J. F., Gordon P. L., et al. Design of an artificial skin: II. Control of chemical composition. J. Biomed. Mater. Res. 1980; 14: 107–131
- Yang H., Zhang Q. Preparation and characterization of collagen-GAGs bioactive materials for tissue engineering. J. Mater. Sci. Technol. 2001; 17(5)495–500
- Osborne C. S., Barbenel J. C., Smith D., et al. Investigation into the tensile properties of collagen/chondroitin-6-sulphate gelsX the effect of cross-linking agents and diamines. Med. Biol. Eng. Comput. 1998; 36(1)129–134
- Lopez Valle C. A., Germain L., Rouabhia M., et al. Grafting on nude mice of living skin equivalent produced using human collagens. Transplantation 1996; 62: 317–323
- Gratzer P. F., Pereira C. A., Lee J. M. Solvent environment modulates effects of glutaraldehyde crosslinking on tissue-derived biomaterials. J. Biomed. Mater. Res. 1996; 31: 533–543
- de Vires H.J. C., Mekkes J. R., Middkoop E., et al. Dermal substitutes for full thickness wounds in a one stage-grafting model. Wound Repair Regener. 1993; 1: 244–252
- Hansbrough J. F., Morgan J., Greenleaf G., et al. Development of a temporary living skin replacement composed of human neonatal fibroblasts cultured in biobrane, a synthetic dressing material. Surgery 1994; 115: 633–644
- Middlekoop E., de Vries H.J. C., Ruuls L., et al. Adherence, proliferation and collagen turnover by human fibroblasts seeded into different types of collagen sponges. Cell Tissue Res. 1995; 280: 447–453
- Yannas I. V., Lee E., Orgill D. P., et al. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. U. S. A. 1989; 86: 933–937