INTRODUCTION
Since cells are the fundamental units of all tissues and organs, what are the possibilities of using the principle of artificial cells for the construction of artificial organs? The idea of artificial cells has been applied to the design and construction of compact artificial kidneys (Chang, 1966), with the hope that this can be used as a feasibility test for the construction of other types of artificial organs. Part of this system is now being tested clinically with promising results (Chang and Malave, 1970; Chang et al., 1971).
CONVENTIONAL ARTIFICIAL KIDNEYS
Before discussing the details of artificial kidneys constructed from artificial cells, a brief summary of the basic functional characteristics of the conventional artificial kidneys should be given. It is obviously not within the scope of this monograph to review meaningfully the large amount of work on artificial kidneys, nor is it possible to include references for all the many publications on this subject. A few of the many excellent publications include monographs (Kolff, 1946; Schreiner and Maher, 1961; Doyle, 1962; Merrill, 1965; Hampers and Schupak, 1967; Brest and Moyer, 1967; Nose, 1969), journals (Schreiner, 1955–1970); Kerr, 1967–1970), reviews (Leonard, 1966; Dedrick et al., 1968), and reports (Burton, 1967; Gottschalk, 1967).
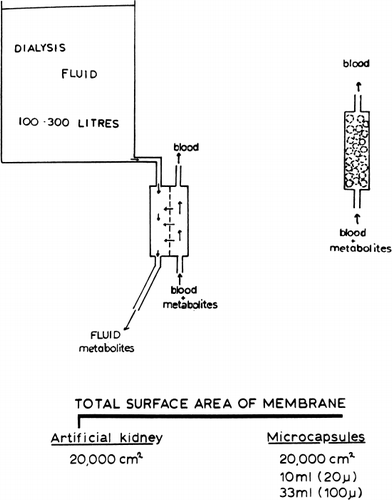
In 1913, Abel et al. devised a hemodialysis apparatus to remove toxic materials in experimental animals. Kolff and Berk in 1943 developed the rotating drum artificial kidney and demonstrated for the first time the clinical usefulness of hemodialysis. With the development of the Scribner-Quinton arteriovenous shunts (Quinton et al., 1960), long-term hemodialysis became a reality. Other centers, realizing the importance of these findings, have placed increasing emphasis on the development and uses of hemodialysis. At present, different types of artificial kidneys are being used. These include the coil type, e.g. Kolff twin coil artificial kidney (Kolff and Watschinger, 1956); Alwall coil artificial kidney (Alwall, 1947); the plate type, e.g. Skeggs and Leonards's (1948) artificial kidney; the Kill (1960) type artificial kidney; and more recently, the capillary type (Stewart et al., 1966). All these artificial kidneys have in common the following basic principle (): a semipermeable membrane separates two compartments. The patient's blood flows through one compartment. Here, permeant molecules from the patient's blood cross the semi-permeable membrane by diffusion along electrochemical and hydrostatic gradients to the other compartment where they are washed away by a very large volume of dilaysate (200–300 liters). The rate of removal of waste metabolites depends on a number of factors. The membrane thickness and total membrane area available for diffusion are two of the factors. The success of this basic principle is clearly demonstrated by the large number of chronic renal failure patients successfully maintained by chronic hemodialysis. Despite the therapeutic value of hemodialysis, Professor Kolff has recently quoted that only 1.5 percent of the people who need it are being helped with artificial kidney or kidney transplantation (Nose, 1969). In other words, in the United States alone, out of 40,000 people who require treatment only about 500 can be accommodated. Serious attempts are being made by many centers to construct simpler and less costly artificial kidneys.
THEORETICAL ASPECTS OF ARTIFICIAL KIDNEYS BASED ON ARTIFICIAL CELLS
The basic principle of the use of artificial cells for the construction of compact artificial kidneys was first proposed in 1966 (Chang, 1966) (see ). It is well known that the smaller a particle, the larger is the surface area to volume relationship. If we calculate the total surface area in artificial cells of different diameters, the result obtained is quite striking. Thus, 10 ml of 20μ diameter artificial cells or 33 ml of 100μ diameter artificial cells has a total surface area about 20,000 cm2, which is larger than that available in a conventional artificial kidney (less than 20,000 cm2). In addition, the membrane thickness of artificial cells (less than 500 Å) is much less than that used in standard artificial kidneys (at least 50,000 Å). If everything else remains the same, the rate of net movement of permeant molecules across a membrane (ds/st) along a given concentration gradient (ΔCs) is proportional to the surface area (A) and inversely proportional to the membrane thickness (dx), sinceThe principle of the construction of a compact artificial kidney from semipermeable artificial cells is simply as follows: If we pack 33 ml of 100μ diameter artificial cells in a shunt, then the total membrane area available for diffusion will be greater than that of an artificial kidney. Since the membrane thickness is at least two orders of magnitude less than that in the standard artificial kidney, metabolites from blood flowing past these artificial cells can cross the membrane into artificial cells at least 100 times faster than in standard artificial kidneys. If something can be placed inside these artificial cells to trap or act on the metabolites which cross the membrane, then we have the theoretical basis for a small compact artificial kidney. For this model to become practical, we have to answer the following questions: What are the permeability characteristics of the artificial cell membrane? What is the compatibility of artificial cells with blood? What can be included within the artificial cells to trap waste metabolites?
The permeability characteristics of artificial cells have been studied in detail (Chang, 1965; Chang and Poznansky, 1968c). As discussed in the earlier chapter, the equivalent pore radius of the artificial cells can be varied at will over a wide range. Those prepared by the standard procedures (Chang, 1964; Chang et al., 1966) have an equivalent pore radius of 18 Å, a value comparable to that used in dialysis membranes. Thus, the artificial cell membranes are impermeable to particulate matters (e.g. blood cells or suspensions) and macromolecules (e.g. enzymes and other proteins). Smaller molecules, however, can equilibrate rapidly across the membranes. The rate of equilibration of uremic metabolites like urea, creatinine, and uric acid is so rapid that a mixing and sampling procedure is required for the analysis of permeability constants. The results obtained and described in an earlier chapter show that the permeability characteristics of artificial cell membranes are suitable for the rapid permeation of dialyzable uremic metabolites. In addition, the permeability characteristics of artificial cell membranes can be modified by variations in polymer materials, thickness, porosity, charge, lipid coating, and polysaccharide coating.
Results described in the previous chapter indicate that artificial cells can be prepared to have membranes which are compatible with the formed elements of blood. The next question which requires solution is the type of materials for the removal of uremic metabolites.
REMOVAL OF BLOOD UREA
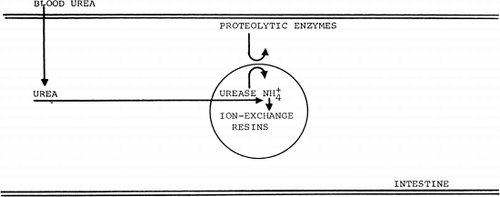
At present, no absorbent is available which has a sufficient absorbing capacity for the removal of blood urea. Studies in this laboratory demonstrated the possibility of using artificial cells containing urease (Chang, 1966).
Procedures and Methods
Control artificial cells containing no urease and urease-loaded artificial cells (soluble urease, Nutritional Biochemical Co., 400 mg/3.0 ml artificial cells) are prepared according to the standard procedure described for nylon artificial cells of 90μ mean diameter (Chang et al., 1966). Before use, Tween 20 must be removed as completely as possible from the artificial cell suspension by washing at least six times on the centrifuge with 10 volumes of saline.
The extracorporeal shunt system I have used in experiments on dogs is shown diagrammatically in Figure 46. The artificial cells (90μ mean diameter, smallest ones removed by differential screening) must be retained in the shunt chamber without stasis or close packing and without interfering with the free perfusion of blood. This is accomplished by placing a 325-mesh nylon screen, supported by a steel wire screen, at either end of the chamber; the distance between the screens is 15 cm and the internal diameter of the chamber is 2.0 cm. With the pump adjusted to give a perfusion rate of 20 ml/min, it is desirable to reverse the direction of flow every 10 minutes to prevent clogging of the screen. The system functions well for three to four hours; for longer periods of operation the shunt chamber needs further modifications, or the perfusion may be switched to a second chamber.
Nembutal-anesthetized dogs with an average weight of 10 kg were used in the acute experiments. Right femoral artery (A) and vein (V) were cannulated and connected to the shunt system (Fig. 46). Each dog received 2.0 mg/kg of aqueous heparin sodium (Nutritional Biochemical Co.) intravenously. In each case, arterial blood was perfused for 90 minutes through the shunt chamber; during this period the chamber contained 10 ml of control nylon artificial cells which were not loaded with urease. Thereafter, arterial blood was perfused for a second 90-minute period through the same chamber, which then contained 10 ml of urease-loaded artificial cells.
Results
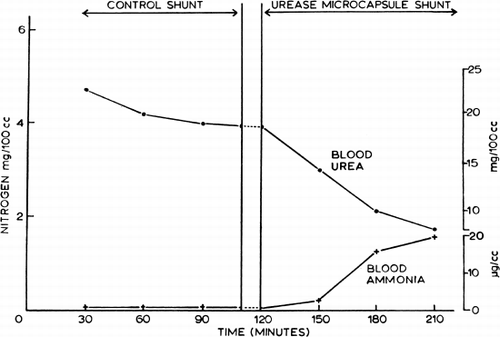
Systemic blood ammonia levels were never significantly changed when arterial blood was perfused at 20 ml/min through a shunt containing control artificial cells. On the other hand, systemic blood ammonia levels were regularly increased when the shunt chamber contained urease-loaded artificial cells. Thus blood ammonia rose steeply, the average level after 90 minutes being 28.5 (range 20.0–34.0)μg/ml (), in spite of the presence in these dogs of a functioning liver which was presumably converting ammonia to urea during the course of the experiment. Ammonia in the effluent was too high and urea in the effluent was too low for satisfactory estimation by the methods routinely employed, but it was clear that at least 80 percent of the urea in the blood perfusing the shunt had been converted to ammonia. The results for ammonia and urea in systemic arterial samples from this experiment are shown in , where the values are also plotted in terms of urea and ammonia N. The rise in blood ammonia N accounted for about three-fourths of the fall in blood urea N; a closer agreement would hardly be expected in view of the likely differences in distribution and in renal and metabolic handling of the two substances.
Figure 58. Extracorporeal shunt containing urease-loaded artificial cells. Effect on systemic arterial blood ammonia and blood urea level. (From Chang, 1966. Courtesy of the American Society for Artificial Internal Organs.)
The urea–urease system has been used here as a model to test the feasibility of using urease-loaded artificial cells to lower blood urea level. From this point of view the results seem promising; the shunt gave more than 80% conversion, though the volume of the incorporated artificial cells was only 10 ml. That ammonia formed from the enzymatic action on urea could be removed by artificial cells containing ammonia absorbent has been demonstrated in the same studies (Chang, 1966). Thus with slight modification of the procedure, nylon artificial cells containing Dowex-X12 can be produced. Shunts have been loaded with artificial cells of up to 120 ml. Over short periods of perfusion such a shunt chamber was effective in removing most of the ammonia from the perfusing blood of animals whose ammonia levels have been raised. For practical use, ammonia absorbents with much higher capacity will be required.
General Discussion
This study demonstrated the theoretical possibilities of using artificial cells containing a combination of urease and ammonia absorbents for the removal of blood urea. Other workers have supported the feasibility of using artificial cells for the removal of urea. Thus, Levine and LaCourse (1967) analysed the theoretical feasibility of a column of artificial cells containing urease to remove blood urea. In this analysis, the extra renal blood volume and urea concentration are represented respectively by VB and UB, and the volume of blood flowing through the artificial kidney per unit time is represented by V. If M is the rate of urea production and Uo the concentration of urea at the efferent of the shunt, then
If f represents the ratio of Uo/UB, then the steady-state solution to the above equation becomes
Thus the steady-state concentration depends on V, the rate of blood through the artificial kidney, on M, the rate of urea production, and on f, the ratio between the urea concentration in the afferent (UB) and efferent (Uo) of the artificial kidney. If f is very much less than 1 (in fact about 0.1, as shown experimentally by Chang, 1966), and knowing M (25 gm/24 hr) and UB (0.25 gm/liter), then the required blood flow rate through the artificial kidney can be estimated at about 70 ml/min.
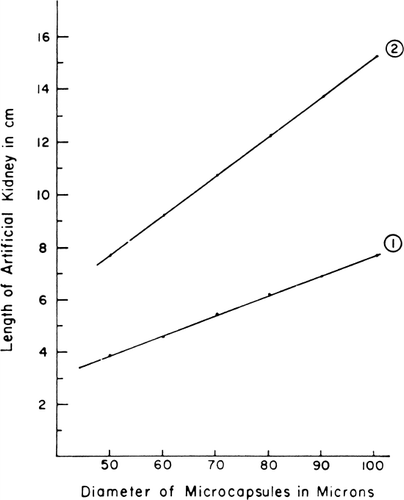
With further derivations taking into account enzyme kinetics, membrane permeability, blood flow rate, and other factors, the authors (Levine and LaCourse, 1967) arrived at the following equation,where h is the length of column required, v is linear velocity of blood, f is ratio of the efferent urea concentration, P is the permeability constant of the artificial cell membrane, F the fraction of the column occupied by artificial cells, and r the radius of the artificial cells. They used this equation to derive a graph () showing column height as a function of arterial cell diameters for two values of the permeability constant. This graph shows that in theory, it should be possible to use artificial cells to construct an artificial kidney 2 cm is diameter and 10 cm in length for the conversion of urea produced daily to ammonia.
Figure 59. The length of column required for f=1/10 is plotted versus artificial cell diameter. P=1×10−4 cm/sec in curve 1, and P=5×10−5 cm/sec in curve 2. Other details are described in the test. (From Levine and LaCourse, 1967. Courtesy of Interscience Publishers, New York.)
Our approach of combining urease and ammonia absorbents for the removal of blood urea has been further extended. It has been suggested (Chang, 1966) that by placing such materials as these in the external dialyzing fluid of an artificial kidney, the urease Dowex system can also act on urea diffusing across the hemodialyzer membranes. The main advantage of enclosing them in artificial cells is the enormous ratio of surface area to volume that may be attained in this way. With artificial cells of 20μ diameter, for instance, the surface area available for exchange is 2,500 cm2/ml of artificial cells. Thus 10.0 ml of such artificial cells have more surface area than a conventional artificial kidney. This large surface area and small volume should be useful in cases where it is desirable to minimize the volume of extracorporeal blood, or where the biological material is expansive (e.g. enzymes), or where a portable or wearable device is wanted. Spark's group (1969, 1971) extended this approach by suggesting the feasibility of artificial cells containing urease and ammonia absorbent or urea absorbent in the gastrointestinal tract or in the dialysis fluid of artificial kidneys for the removal of urea. The use of urease has also been modified by Gordon et al. (1969) for the removal of urea from dialysance. Other workers (Falb et al., 1968; Flinn and Cherry, 1970; Gardner, 1971; Sparks et al., 1971) are investigating the removal of urea by artificial cells containing different systems: urease and ammonia absorbent; urease and another enzyme system; or urea absorbent.
Work in this laboratory suggests the possible gastrointestinal approach for the removal of urea (Chang and Poznansky, 1968a) (Chang and Loa, 1970). In the intestine, bacterial urease converts urea into ammonia which is reabsorbed. Therefore, there is little intestinal excretion of urea or ammonia. However, in the present experiments, four hours after the oral administration of zicronium phosphate (ammonia absorbent), the systemic blood urea levels were 11.0±1.1 mg% as compared to 14.5±1.5 mg% in the control rats. The serum calcium levels were 10.22±0.77 mg% in the treated group and 10.65±0.40 mg% in the control group. Similar results were observed when another ammonia absorbent, Dowex-50W-X12, was administered orally into rats. Within four hours, the systemic blood urea levels had decreased to 65.0±4.5 percent of the control values. When animals were treated with an antibiotic mixture (sulfaguanidine, Terramycin®, penicillin, and neomycin) to eliminate the urease-producing bacteria, oral administration of ammonia absorbents had little effect on the systemic blood urea levels (89.1±6.0 percent of the control values). In these antibiotic-treated rats, the oral administration of both microencapsulated urease and ammonia absorbents lowered the systemic blood urea levels to 60.6±5.0 percent of the control levels within four hours. Thus microencapsulated urease efficiently replaced the bacterial urease activity. In conclusion, a combination of exogenous ammonia absorbents and bacterial or microencapsulated urease in the intestine can efficiently remove systemic urea. More recently, aldehyde starch has been tested for the removal of urea (Giordano and Esposito, 1970; Sparks et. al. 1971) .
REMOVAL OF OTHER UREMIC METABOLITES
What can be placed inside the artificial cells to remove other uremic metabolites entering the artificial cells? While no ideal reactants are as yet available for this purpose, the enclosure of activated charcoal in artificial cells has been proposed (Chang, 1966) and studied in experimental animals (Chang et al., 1967b, 1968, 1970; Chang, 1969e), and in uremic patients (Chang and Malave, 1970; Chang et al., 1971).
It is known that activated charcoal can efficiently remove many uremic metabolites from perfusing blood and improve the symptoms of patients so treated (Yatzidis, 1964; Dunea and Kolff, 1965). Unfortunately, activated charcoal also adversely affects platelets of perfusing blood (Dunea and Kolff, 1965; Dutton et al., 1969) and releases embolising particles (Hagstam et al., 1966). On the other hand, when microencapsulated within semipermeable membranes (Chang, 1966), the activated charcoal is enclosed in an “intracellular environment.” The enclosing membranes prevent any free powder from going into the circulation, and at the same time, prevent the blood platelets or plasma protein from coming into direct contact with the enclosed activated charcoal.
Compact Artificial Kidney System
The use of the standard procedure, as previously described (Chang, 1964; Chang et al., 1966), is not suitable for the microencapsulation of activated charcoal in a scaled-up system. The flexibility of the enclosed membranes results in packing and increased resistance to flow. The formation of artificial cells by polymer coating (Chang et al., 1968; Chang, 1969e) prevents these problems and is the procedure which was used here. In the present study, coconut activated charcoal (6–14 mesh, Fisher Co.) is microencapsulated in albumin-coated collodion membranes (ACAC) by the procedures already reported (Chang, 1969e). After microencapsulation, instead of using the spray-drying procedure, the artificial cells are spread out and allowed to evaporate in a well ventilated 50°C oven until the ether has been removed (5 hours). They are then washed repeatedly with pyrogen-free distilled water through a sieve (40 mesh) untill all free particles which have escaped microencapsulation are removed. In all experiments, and in the clinical trial, all artificial cells were used within 1 month after preparation.
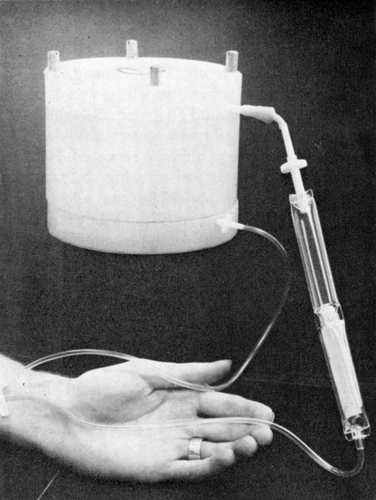
Activated charcoal (300 gm) or artificial cells containing 300 gm of activated charcoal are placed in a silicone-coated (Silicone Spray, Manostat Co.) extracorporeal shunt chamber (10 cm diameter and 8 cm height) prepared as described previously (Chang, 1969e) (). The afferent shunt connection was prepared from sterile tubing (R62A Travenol), and the efferent connection, with air and clot traps, was prepared from a blood administration set (R78 Baxter). The priming volume of the connections and fully loaded shunt is about 300 ml.
Figure 61. Extracorporeal shunt chamber containing 300 gm of ACAC articial cells used for in vivo experiments and for clinical trials. (From Chang et al., 1970. Courtesy of the American Society for Artificial Internal Organs.)
Different types of sterilization procedures, including ethylene oxide, formalin, and autoclaving have been examined as possible sterilization methods for this system. Autoclaving at 121°C, 15 psi has been found to be suitable. Before microencapsulation, activated charcoal granules are washed to remove all fine particles. They are then autoclaved for 60 minutes. After further washing, they are wrapped and sealed in sterile cloth. The sealed portions are then autoclaved for 30 minutes, then allowed to dry completely at 60°C (at least 48 hours). After microencapsulation, evaporation, and the sieving procedure, the microencapsulated activated charcoal is placed in the shunt, primed with water, and autoclaved for 30 minutes. This process may disturb some artificial cell membranes, and careful perfusion at 200 ml/min with sterilized saline (4 liters) is required to remove any possible free particles. This is followed by albumin coating; using sterilized technique, the shunt is filled with 1 gm% human albumin (Cutter Co.), then left standing at 4°C for 12 hours. Just before use, the shunt is perfused with 2 liters of sterilized saline. Heparin (1000 units) is injected into the shunt and 300 ml of saline is displaced by the patient's blood before the effluent is returned to the patient. The patient is heparinized systematically with 100–120 units/kg of heparin. At present, a 200 ml/min blood flow can be obtained without the use of a blood pump if the Scribner type of arteriovenous shunt is available. For the internal A-V fistuli, a blood pump is required. In these cases, a rotary pump and not a Sigma pump should be used.
The internal resistance of 35 mm Hg across the ACAC artificial cell type artificial kidney is low when compared to those of the conventional artificial kidneys: 100–300 mm Hg for the coil type artificial kidneys; 50 mm Hg for the Kiil type; and 45 mm Hg for the Dow type (Cestero and Freeman, 1969).
In Vitro and In Vivo Removal of Uremic Metabolites
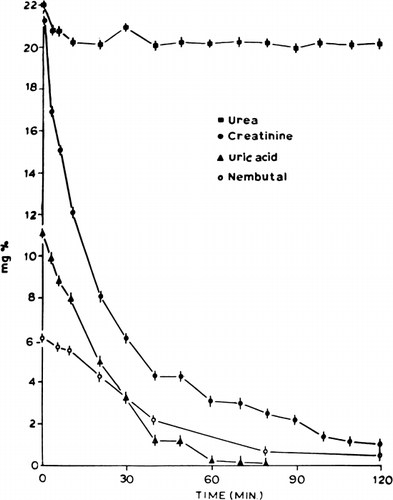
Creatinine. This was studied in detail in this report as a test of the efficiency of the artificial cell system. The in vitro removal of creatinine was first studied using stirred-batch experiments at 37°C. ACAC microencapsulated activated charcoal was added to a well-stirred aqueous creatinine solution (21 mg%) in amounts of 1 gm/30 ml of solution. The concentration of creatinine decreased at a rapid rate (); 50 percent of the creatinine was removed within 12 minutes.
Figure 62. Rate of decrease in concentration of different solutes in stirred batch experiments (equivalent to 300 gm ACAC artificial cells in 9 liters of solution). (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
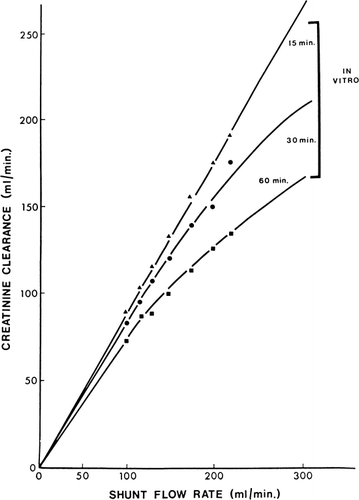
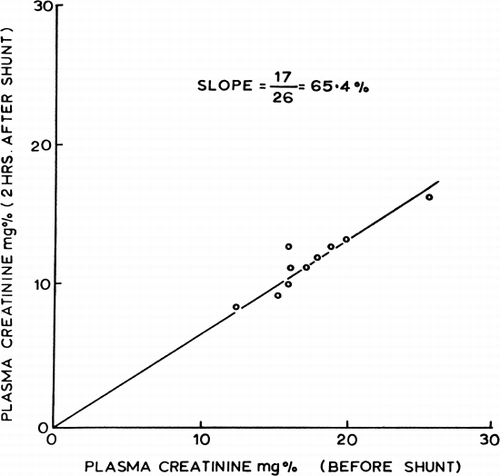
Next, 300 gm of ACAC microencapsulated activated charcoal was used in single pass studies in which the creatinine concentration entering the shunt was kept at 20 mg%. shows the flow rates and time of perfusion. Creatinine clearance was calculated from the difference in creatinine concentration entering and leaving the shunt and the shunt flow rate. The results obtained are shown in and compared to other types of artificial kidneys in . These studies show a higher creatinine clearance for 300 gm of ACAC microencapsulated activated charcoal in vitro than for the best conventional artificial kidneys ().
Figure 63. In vitro creatinine clearance of a shunt containing 300 gm of ACAC artificial cells. (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
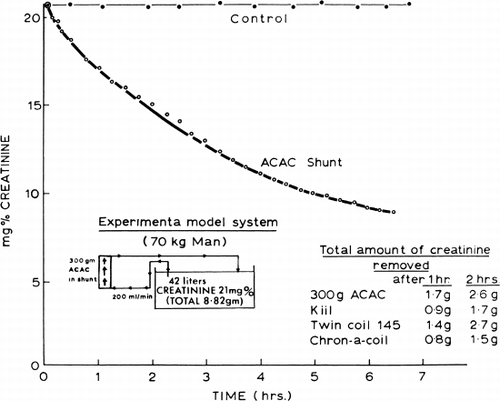
Further in vitro studies were done using a bath containing 42 liters of creatinine solution (21 mg%) (). Bath solution was recirculated continuously through a shunt containing 300 gm of ACAC microencapsulated activated charcoal. Within two hours the level of creatinine in the bath solution was lowered by 25 percent, representing the removal of 2.2 gm of creatinine within this period of time.
Figure 64. Schematic representation of the removal of creatinine by 300 gm of ACAC artificial cells.
In vivo experiments in 20 bilaterally nephrectomized dogs were done (). The experimental procedures described earlier (Chang, 1969e) were used. Briefly, arterial blood from each Nembutal-anesthetized dog (20–27 kg) was perfused through a shunt containing 300 gm of microencapsulated activated charcoal. A flow rate of 120 ml/min was possible without the use of blood pumps. shows the results obtained in 10 dogs treated with 300 gm of ACAC (albumin-coated collodion microencapsulated activated charcoal). In all cases () the decrease in arterial creatinine level after two hours of hemoperfusion was about 35 percent; with a higher blood flow of 300 ml/min, the decrease was 75 percent. The decrease was most rapid in the first 30 minutes of hemoperfusion. compares the results obtained using albumin-coated collodion microencapsulated activated charcoal (ACAC) with those using free activated charcoal (AC) and those using collodion microencapsulated charcoal (CAC). ACAC and CAC microencapsulated activated charcoal removed plasma creatinine at a rate which was only slightly slower than free activated charcoal.
Figure 65. Rate of change of plasma creatinine levels in nephrectomized dogs treated with hemoperfusion (blood flow 120 ml/min) through free AC activated charcoal, CAC artificial cells, and ACAC artificial cells; 300 gm in each shunt. Lower curve (○) represents rate of change of plasma creatinine with higher blood flow (300 ml/min) through 300 gm of ACAC artificial cells. (From Chang and Malave, 1970. Courtesy of American Society for Artificial Internal Organs.)
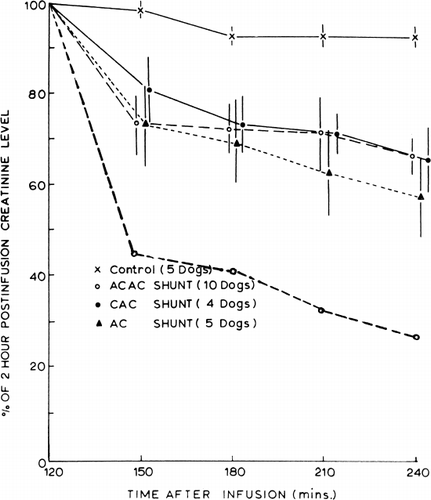
Figure 66. Effects of two-hour hemoperfusion through 300 gm of ACAC artificial cells on plasma creatinine levels of 10 nephrectomized dogs. (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
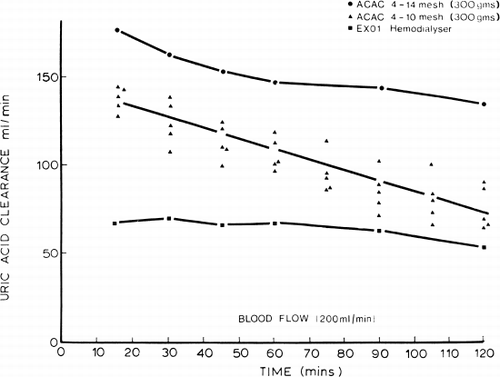
Uric acid. shows the result of stirred-batch experiments. Uric acid was removed efficiently by ACAC microencapsulated activated charcoal in vitro. Thus, 300 gm of this material removed 50 percent of the uric acid in 9 liters of an 11 mg% solution within 18 minutes. In vivo experiments in five dogs also showed that uric acid was removed efficiently.
Urea, Ca++, phosphate, albumin, and total protein. As shown in , ACAC microencapsulated activated charcoal only removed a very small amount of urea. In vivo experiments showed, as in the case of free activated charcoal, that ACAC microencapsulated activated charcoal had little effect on the removal of urea, Ca++, phosphate, plasma albumin, or total protein.
Guanidine. Guanidines are efficiently removed by artificial cells containing activated charcoal.
Clinical Tests for Safety of the System
Experiments described in the last chapter showed that albumin-coated artificial cells containing activated charcoal did not have any adverse effects on the formed elements of blood; no emboli were observed, and animals continued to do well in long-term studies. Thus at the Royal Victoria Hospital we initiated clinical trials of this system for chronic renal failure (Chang et al., 1970). The first clinical trials were to test the safety of the system, and subsequent trials were to test the efficiency of the system. The first clinical test is summarized below ():
Figure 67. Response of patient (B.B.) treated with hemoperfusion through 300 gm of ACAC artificial cells. (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
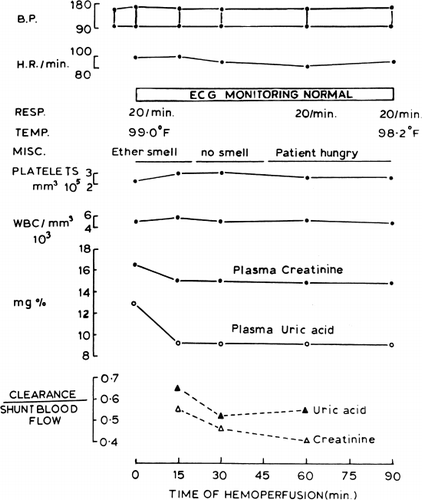
B.B. is a 50-year-old white male with chronic renal failure and chronic lung disease. The patient could not be accommodated in either a chronic hemodialysis or a renal transplantation program and had been maintained by peritoneal dialysis. On the day of the clinical trial, his biochemical data were as follows: BUN 102 mg%, creatinine 16.5 mg%, uric acid 12.9 mg%, sodium 143 mEq/litre, chloride 98 mEq/litre, potassium 4.4 mEq/litre, calcium 7.4 mg%, and phosphorus 6.4 mg%.
The extracorporeal shunt chamber containing 300 gm of albumin-coated collodion microencapsulated activated charcoal (ACAC) was used (). Sterilization of the system was described. After percutaneous puncture of the left femoral artery (14-gauge catheter), 3000 U.S.P. units of heparin was injected into the shunt and 5000 U.S.P. units intravenously into the patient. Blood from the left femoral artery entered the shunt to displace the 350 ml saline priming solution. After discarding the 350 ml priming solution, the effluent flow from the shunt was returned to the patient by the cephalic vein (14-gauge catheter). No blood pump was used, and a shunt blood flow of 100 ml/min was maintained.
At the beginning of the hemoperfusion procedure, the patient stated that there was a smell of ether. It was found that this came from a trace amount of residual ether present in the collodion membranes. This smell disappeared shortly. The patient felt well throughout the 90-minute procedure. No other side effects were noted. Instead of nausea and vomiting encountered by some patients treated with free activated charcoal hemoperfusion (Dunea and Kolff, 1965) our patient started to feel hungry halfway through the procedure. The patient accepted this procedure so completely that immediately after the procedure, he agreed to have repeated hemoperfusion in the future. The patient was followed for 48 hours with no adverse effects and was discharged from the hospital. He is scheduled for further hemoperfusions. Since then, the patient has received another hemoperfusion procedure with no adverse effects.
Hematological data from this laboratory showed that 90 minutes of hemoperfusion did not result in any adverse effects on platelets (237,000/mm3 initial level to 234,000/mm3 after hemoperfusion); neither leucocytosis nor leucopenia was noted (5,170/mm3 initial level to 4,290/mm3 after hemofusion); and there was no significant increase in the plasma hemoglobin level.
Performance Characteristics in Clinical Trials
The results obtained in 51 clinical tests (Chang et al., 1971) are summarized below:
Internal resistant. The internal resistance measured as pressure drop across the microcapsule artificial kidney is very low (). At a blood flow rate of 200 ml/min the internal resistance of the artificial kidney prepared from the 6–10 mesh microcapsule (25 mm Hg) is less than that prepared from the 6–14 mesh microcapsules (45 mm Hg). In both cases, the internal resistances are less than the Kiil-type hemodialyzer (50 mm Hg). This internal resistance is extremely low when compared to the coil-type hemodialyzer (mostly over 150 mm Hg). Thus, in patients with Scribner A-V shunts, a blood flow rate of 150–260 ml/min can be obtained with the microcapsule artificial kidneys without the use of a blood pump. In those patients with internal A-V fistuli, blood flow was much slower.
Figure 73. Internal resistance and creatinine clearance of microcapsule (ACAC) artificial kidney compared to the values obtained by Cestero et al. for other hemodialyzer. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
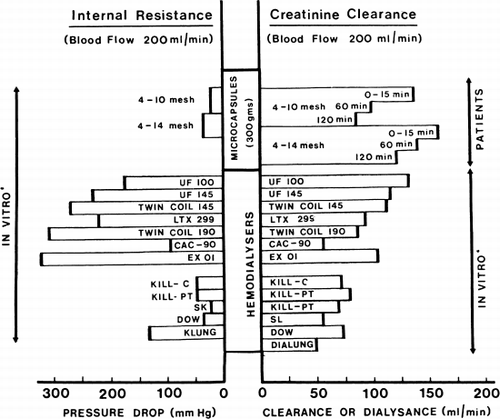
Creatinine. In vitro studies show that at a flow rate of 200 ml/min, 300 gm of albumin-coated microencapsulated activated charcoal can remove 5 gm of creatinine. The result of the removal of creatinine in the 51 clinical trials is summarized in and . It is clear that within a two-hour hemoperfusion period, the creatinine clearance of 300 gm of albumin-coated microencapsulated activated charcoal is comparable to the most efficient types of coil artificial kidney and much more efficient than the plate-type or capillary-type artificial kidneys.
Figure 68. Creatinine clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in 15 clinical trials. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
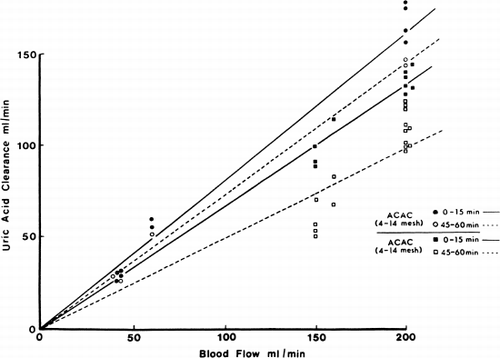
Uric acid. The uric acid clearance in the 51 clinical trials is shown in and . It is clear that uric acid clearance is even more efficient than the standard hemodialyzers.
Figure 69. Uric acid clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in 15 clinical trials. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
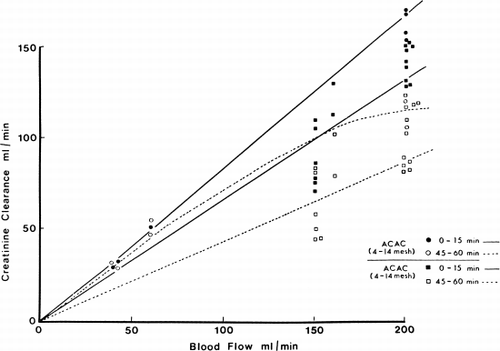
Figure 70. Creatinine clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in clinical trials compared to those obtained using EX01 hemodialyzers. Blood flow rate 200 ml/min. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
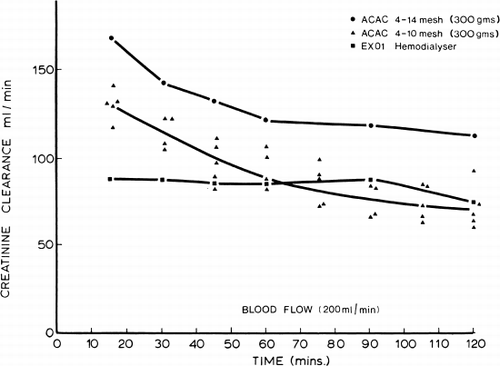
Figure 71. Uric acid clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in clinical trials compared to those obtained using EX01 hemodialyzers. Blood flow rate 200 ml/min. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
Guanidine. In vitro studies show that guanidine can be removed efficiently by microencapsulated activated charcoal. Pre- and post-hemoperfusion values for guanidine were measured in four clinical trials. In one of these, the analysis was interfered by a very high blood urea level. The results of the three successful analyses are shown in . It is shown that guanidine, an important uremic metabolite, can be efficiently removed.
Table 5. Removal of Guanidine
Urea, water, and electrolytes. The 300 gm of microencapsulated activated charcoal only removed a small fraction of blood urea. Other systems are being developed for the removal of urea (Chang, 1966; Levine and LaCourse, 1967; Chang and Loa, 1970; Falb et al., 1970; Sparks et al., 1971; Gardner, 1971). Microencapsulation of possible urea absorbents is also being investigated (Sparks et al., 1971). The present microencapsulated activated charcoal system does not contribute to the removal of sodium, potassium, phosphate, or water. Microencapsulation of ion-exchange resins (Chang, 1966) is being investigated.
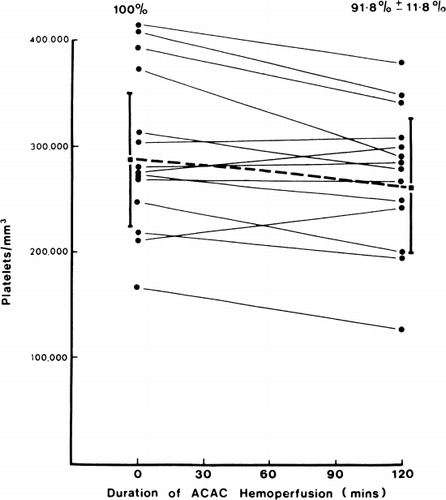
Effects on platelets and safety of system. The posthemoperfusion level in the case of albumin-coated microencapsulated activated charcoal was 91.8%±11.8% of control values (). This is a negligible change when compared to the 50% post-hemoperfusion level in uncoated activated charcoal (Dunea and Kolff, 1965; DeMyttenaere et al., 1967; Hagstam et al., 1966; Dutton et al., 1969) and the 70% posthemodialysis level in coil hemodialyzer (Lawson et al., 1966). Smear of shunt effluent blood did not show any embolic particles. These observations in clinical trials confirm our earlier findings that hemoperfusion over albumin-coated microencapsulated activated charcoal did not give rise to embolising particles or changes in platelet levels (Chang, 1969; Chang and Malave, 1970).
Figure 72. Effects of hemoperfusion over albumin-collodion coated activated charcoal on patients' arterial platelet levels. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
Transient pyrogenic reactions were observed in two of the earlier hemoperfusions. The blood cultures were all negative. Pyrogenic tests showed that the albumin-coated microencapsulated activated charcoal was not pyrogenic. The most likely cause may have been due to the reuseable screens of the shunt chambers. Since then, careful washing of the reusable shunt chambers with sodium hypochlorite to remove any protein deposited in the wire meshes and elsewhere has eliminated the problems of pyrogenic reactions. No other side effects were observed.
Chronic Intermittent Hemoperfusions with the Microcapsule Artificial Kidney
The microcapsule artificial kidney is at present being used for maintainance chronic hemoperfusion (Chang et al., 1971). The patient is a 71-year-old Italian woman with chronic renal failure and congestive heart failure. Her general condition was such that she was not suitable for either the transplantation or chronic hemodialysis program. After peritoneal dialysis, she was placed on medication and diet. The patient continued with her medication and diet but did not return for her subsequent peritoneal dialysis. She was admitted to the hospital three months later with complaints of nausea, vomiting, low back pain, diarrhea, and hiccups. Her biochemical data at the time of admission was as follows: BUN 186 mg%, creatinine 24.9 mg%, Na 142 mEq/litre, K 5.8 mEq/litre, and C1 102 mEq/litre. Her urinary output was about 600 ml/24 hours and creatinine excretion was about 80 mg/24 hours. She was placed on a regime of intermittent microcapsule hemoperfusions supplemented with hemodialysis as required. In addition, she continued her medication of Gravol, Amphojel®, folic acid, Kayexalate®, sodium bicarbonate, and Bemianl® with C fortis; and her restriction to 40 gm protein, 40 mq Na and 40 mq K. The hemoperfusion and hemodialysis schedule and the biochemical data is shown in . Throughout these hemoperfusions, the patient did not experience any pyrogenic reactions or other side effects. During two of the 28 hemoperfusions, there was a slight decrease in her blood pressure (130/80 to 110/70) during the procedure. However, her blood pressure immediately returned to her preperfusion levels at the completion of the two-hour procedure when the extracorporeal blood in the chamber was reinfused into the patient. As shown in , the changes in posthemoperfusion platelet levels are negligible. Furthermore, her systemic platelet levels show a steady increase after the initiation of this regime, (). There is no hemolysis or changes in her plasma hemoglobin levels. Her urinary output remains at 200–600 ml/24 hours. She is doing well after 28 treatments in 8 months.
Figure 74. Patient treated with chronic intermittent hemoperfusion with microcapsules artificial kidney over a two months period. (From Chang et al., 1971. Courtesy of the American Society for Artificial Organs.)
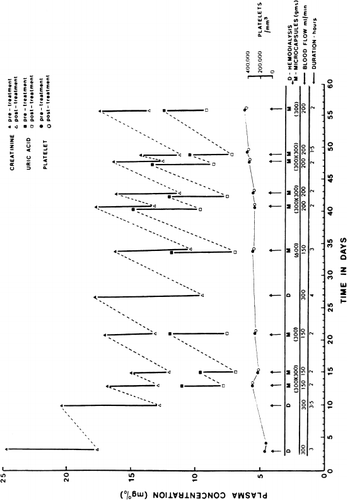
The creatinine and uric acid clearance obtained are shown in , and , along with the other clinical tests, and compared with the standard hemodialysis in . Both systemic creatinine and uric acid levels fell after each hemoperfusion (). The decrease depends on blood flow rate and amount of microcapsules used and ranged from 3.0 mg% to 5.0 mg% in two hours with a hemoperfusion rate of 150 ml/min to 200 ml/min over 300 gm of microcapsules. In the one case where two 300 gm microcapsules shunts were used one after another at a hemoperfusion rate of 150 ml/min, there was a decrease of about 7.0 mg% within three hours. This rate of decrease of systemic creatinine level is more efficient than that of the EX01 hemodialyzer when calculated for the same blood flow rate. Her arterial blood guanidine levels were successfully measured in two of the clinical tests. With two hours of hemoperfusion at 150 ml/min over 300 gm of microcapsules, the arterial level fell from 1.6 mg% to a posthemoperfusion level of 0.4%. With three hours of hemoperfusion at 150 ml/min over 600 gm of microcapsules, the arterial level fell from 2.2 mg% to a posthemoperfusion level of 0.8 mg%. While discussing the removal of guanidine, it is interesting to note that with the present regime of intermittent hemoperfusion, medications and diet, the patient has remained relatively asymptomatic for the last 8 months. She is more active and her appetite has increased and she only returns to the hospital for her hemoperfusion and 2 hours posthemoperfusion observations. Her problem is water retention and ascite. More detailed studies will be carried out in this and other patients to establish the optimal frequency and duration of intermittent microcapsule hemoperfusions and the precise effect of hemoperfusion on the patient's symptomatology.
DISCUSSION
Detailed in vitro, in vivo and clinical results indicate that activated charcoal enclosed in artificial cells is prevented from adversely affecting the formed elements of blood and from giving off massive emboli. The clearance of creatinine and uric acid by 300 gm of ACAC artificial cells is much more efficient than the conventional artificial kidneys (). The compactness, the absence of a large volume of dialysate fluid, and the ease of operation all favor the suggestion of using artificial cells for the construction of compact artificial kidneys (Chang, 1966). There are other substances such as urea, electrolytes, and water which require removal. Work on the possible uses of microencapsulated urease absorbents or of urease and ammonia absorbents for the removal of urea is in progress () (Chang, 1966, 1970; Gardner et al., 1971; Sparks et al., 1969). Microencapsulated ion exchange resins have been prepared (Chang, 1966); selection of the appropriate resins may allow the removal of selected electrolytes. Even before these can be developed for clinical use, the microencapsulated activated charcoal system can be supplemented by less frequent conventional dialysis. In addition, the microencapsulated activated charcoal can be a simple and useful means of treating acute intoxication.
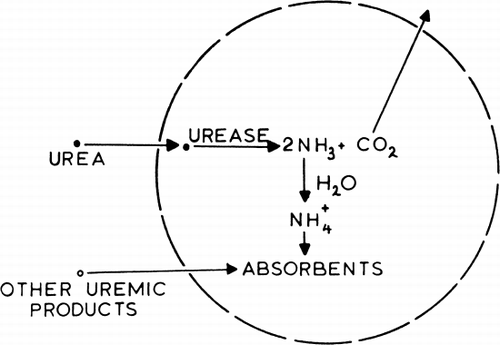
Figure 75. Schematic representation of an artificial cell containing urease and absorbents. (From Chang, 1969. Courtesy of Science Tools, Sweden.)
It should be emphasized that even though the present ACAC artificial cell system is compact, theoroeically it can be made much smaller, e.g. 10 ml (Chang, 1966) or 30 ml (Chang, 1970). Attaining this miniature size, however, would depend on the development of an ideal high capacity absorbent, or discovery of the unknown uremic toxin(s) which can then be selectively removed. The present system requires heparinization. The development of a nonthrombogenic system would greatly simplify the whole procedure. Work on the use of nonthrombogenic artificial cells is already in progress (Chang et al., 1967b, 1968; Chang, 1969e) with promising results. The ultrathin membranes of the ACAC artificial cells withstand gravitational flow, blood flow, and rotatary blood pump flow of 200 ml/min or more, but vigorous pulsatile pumping with the sigma blood pump over long periods can affect the membranes.
The general idea of artificial cells (Chang, 1964, 1966) should not be confined to the construction of artificial kidneys. The present system is a feasibility test to study the use of artificial cells for the design of artificial organs. As such, the results obtained encourage one to proceed further. In nature, cells are the basic unit structures of all organs and tissues. It is left to one's imagination to extend this further into their possible uses to support hepatic failure and other metabolic disturbances. In fact, experiments have shown the possible application of this principle both in enzyme replacement for enzyme deficiency diseases (Chang and Poznansky, 1968a; Chang, 1969d) and in the treatment of asparagine-dependent lymphosarcoma (Chang, 1969c). It is hoped that the basic idea of “artificial cells” will be explored and developed further for the construction of artificial organs. The combination of the capillary dialysis system and the artificial cells being studied in this laboratory is a further step towards this goal.