Abstract
Analogous to the artificial kidney there is a need for an effective and safe liver support system to bridge patients with hepatic failure to liver transplantation or own liver regeneration. An overview is given of the biological and non‐‐biological systems used in clinical practice in the past and at present. The conclusion is drawn that only the biological systems might have the potential to prolong life significantly in patients with acute liver failure. The systems with this potential are summarised. Both in Europe and the USA good bioreactors are available. Most of them are based on porcine hepatocytes, which have immunological and zoonotic drawbacks. What is missing is the well differentiated human hepatocyte in sufficient amounts. Successful development of this cell will be the crown on bioartificial liver research in the third millenium.
Background
Analogous to the artificial kidney there is a need for an effective liver support system to bridge patients with hepatic failure to liver transplantation or to own liver regeneration. In addition, artificial liver support might improve quality of life in patients with end stage liver cirrhosis. It is thinkable that chronic intermittent treatment of cirrhotic patients by such a device will reduce symptoms of tiredness, itching and hemorrhagic diathesis and will improve jaundice and severity of hepatic encephalopathy. Attempts to reach these goals by haemodialysis, haemoperfusion or plasma exchange have been disappointing, although incidentally some improvements have been obtained ((Sussman, [Citation1996])). This was reason to develop better techniques of dialysis or plasma purification devices like diabsorption ((Biologic DT)), ((Ash et al., [Citation1992])) or molecular adsorbance recirculation systems like MARS ((Stange et al., [Citation1999])). Since, in contrast to the kidney, the detoxification activity of the liver, is in particular focussed at protein‐‐bound and lipid soluble compounds these newer dialysis techniques have included albumin adsorption in their system. However, it is generally assumed that a real effective artificial liver should be based on the capacity to perform the liver's multiple synthetic and metabolic functions, including detoxification and excretion ((Sussman et al., [Citation1994])). Hybrid bioartificial systems based on the presence of active functioning hepatocytes in an extracorporeal device that can be connected to the circulation of the patient with liver failure are promising to meet these criteria. The heart of such a system consists of a bioreactor with a sufficient amount of well nourished and oxygenated viable hepatocytes immobilised on a mechanical support (()).
Figure 1. Schematic drawing of the extracorporeal circuit indicating the plasmaferesis device and the BAL perfusion unit.
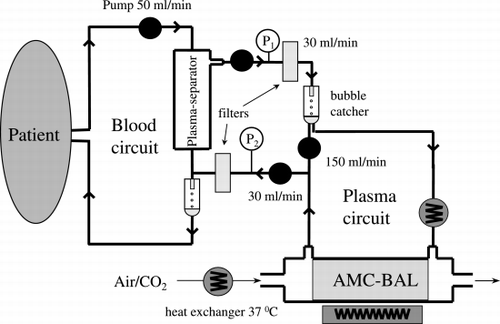
This paper summarises the different used bioartificial systems at present and their in vivo results in patients. For results of the more “classical” blood or plasma purification systems like cross circulation, exchange blood transfusion, plasma exchange, peritoneal dialysis haemodialysis and haemoperfusion the reader is referred to literature All of them have been used in the past to treat acute or subacute liver failure. None of them have shown in a controlled trial a significant improvement of survival ((Chamuleau, [Citation1979]; O'Grady et al., [Citation1988]; Riordan and Williams, [Citation2000]; Sussman, [Citation1996]; Tygstrup et al., [Citation1997])). The same holds for the use of isolated allogenic or xenogeneic isolated liver perfusion ((Sussman, [Citation1996])).
Bioartificial Liver Support Systems
Extracorporeal isolated allogenic or xenogeneic liver perfusion has been used incidentally, but no controlled trials are available.
Most bio‐‐artificial systems use as a biocomponent viable hepatocytes, either freshly isolated primary hepatocytes or a hepatoma cell line.
Several variations of hepatocyte bioreactors have been developed and can be classified according to their culture technique. The hepatocytes may be:
encapsulated in gels
immobilised on collagen coated flat plates
cultured in‐‐or outside semipermeable hollow‐‐fibers ((directly or attached to microcarriers)).
cultured within a porous three dimensional matrix, allowing direct plasma perfusion conditions of the cells.
Ad 1. Encapsulation of liver cells has been successful by the use of alginate. Besides direct cell transplantation of encapsulated hepatocytes in the peritoneal cavity of experimental animals ((Sarkis et al., [Citation1998])), bioartificial livers for extracorporeal use have been developed ((Dixit and Gitnick, [Citation1995])). This development is still in the experimental phase and proof of principle in experimental acute liver failure has to be obtained before clinical application will be justified.
Ad 2. In 1988 Uchino ((Uchino et al., [Citation1988])) published his BAL based on freshly isolated canine hepatocytes attached to collagen coated glassplates. Application of this BAL to anhepatic dogs showed prolonged survival in a series of 4 dogs (()). As we know now, a too small number of animals to obtain significancy due to the large biological variation of such a model.
Table 1. Bioartificial liver support in large animals with acute liver failure
In addition his device was not very practical and no follow‐‐up of clinical application has been published until now.
Ad 3. The principle of the hollow fibre based artificial kidney has been so far the most successful. Blood or plasma is passed through the intracapillary lumen while hepatocytes are attached to the outside of the semipermeable fibre membrane. Interaction between the cells and the plasma of the patient can occur through the semipermeable membrane ((Dixit, [Citation1998])). The Gerlach type of hollow fibre based BAL uses a network of fibres including capillaries meant for oxygenation of the cells at site ((Gerlach, [Citation1996])). Others ((Rozga et al., [Citation1993])) use an extra oxygenator of the plasma before entering the BAL. Besides attachment of the liver cells to the outside of the hollow fibres, cells can also be cultured inside the lumen by using collagen gel as a contracting cell matrix. Plasma is now flowing along the outside of the hollow fibre ((Nyberg and Shatford, [Citation1993])). However, all these hollow fibre devices have the disadvantage of indirect contact between plasma and liver cells in contrast to the in vivo plasma perfusion of the hepatocytes by the sinusoids and space of Disse.
Again significant life‐‐prolonging efficacy in experimental animal models of acute liver failure has only been shown by a minority of them as is shown in . Not all studies have looked at the effect on survival and not all studies have used a sufficient number of animals to allow valid statistical evaluation.
Different experimental animal models of sever acute liver failure have been used: the so called surgical models like complete liver ischemia or total hepatectomy or the chemical ones like galactosamine, acetaminophen or dimethylnitrosamine intoxication.
Ad 4. Others ((Naruse et al., [Citation1998])), including ourselves ((Flendrig et al., [Citation1999a])), have used quite a different way of cell attachment in the bioreactor. Cells are attached to a non‐‐woven poly‐‐ester fabric as a matrix. This matrix is either enclosed as different pieces in a cartridge or as a spirally wound tissue. In the AMC‐‐BAL the space between the different layers is filled with polypropylene oxygen fibres to provide at site an optimal oxygenation of the cells inside the bioreactor.
The big advance of these types of BAL's is the direct contact between plasma and the cells like the in vivo situation. Furthermore, this type of devices can easily contain a very large number of cells ((in the AMC‐‐BAL more than 20 billion of cells)), in contrast to most hollow fibre ones ((with as a positive exception the Berlin type)).
The AMC‐‐BAL was the first to show significant prolongation of survival both in small ((Flendrig et al., [Citation1999a])) and large animals with severe hepatic failure (()) ((Jauregui et al., [Citation1995]; Kelly et al., [Citation1992]; Sheil et al., [Citation1998]; Sielaff et al., [Citation1995])).
Several of the above mentioned BAL's have been tested in the clinic; mostly in uncontrolled studies. The only exception is the pilot controlled study of Ellis et al ((Ellis et al., [Citation1996])), which uses a hollow fibre BAL filled with a human hepatoma cell line.
The results are shown in ((Donini et al., [Citation2000]; Ellis et al., [Citation1996]; Kardassis et al., [Citation1999]; Patzer et al., [Citation2000]; Sussman et al., [Citation1994]; Watanabe et al., [Citation1997])).
Table 2. Clinical results of BAL treatments
Larger controlled trials are under way by the Demetriou BAL and the AMC‐‐BAL.
Future
It is well realised that the use of primary porcine hepatocytes in the BAL has major disadvantages: first, there are enormous immunological problems, even life threatening, to be anticipated by prolonged ((more than 1 week)) use, because of the risk of severe anaphylactic reactions of the recipient to porcine antigens like “serum sickness” ((Te Velde et al., [Citation1997](( and second, the risk of transfection of porcine endogenous retroviruses ((PERV)) to the human population can not completely be excluded ((Patience et al., [Citation1997])). However, until now no direct evidence of such a transfection has been obtained ((Paradis et al., [Citation1999])).
The ideal solution will be the availability of sufficient amounts of viable well differentiated non‐‐tumorigenic human liver cells. In different centers research to obtain these cells is in progress. Potential approaches are genetic engineering of adult liver cells ((Kobayashi et al., [Citation2000])) or manupilation of pluripotent stem cells in the direction of liver cells ((Kubota and Reid, [Citation2000])).
At present, as long as these cells are not yet available, porcine hepatocytes are second best. Clinical immunological experience with xeno‐‐bioreactors in a short‐‐term ((less than 1 week)) phase 1 study in patients has, so far, been reassuring ((Baquerizo et al., [Citation1997])).
Unsolved Problems
Characteristic for all BAL‐‐systems is the lack of a bile excretory system. We have shown ((Flendrig et al., [Citation1997])) that porcine hepatocytes cultured in our bioreactor form bile canaliculi‐‐like structures. This implies that conjugated bile‐‐acids and bilirubin can be excreted into the circulation during in vivo application. This may result in high levels of bile‐‐salts, which might limitate the long term function of the hepatocytes in the bioreactor ((Pazzi et al., [Citation1997])). A possible solution could be the introduction of anion exchange resins and//or silica‐‐based strong anion exchange cartridges for selective adsorption of bile acids and bilirubin ((Morimoto et al., [Citation1989])).
As already indicated above the use of a xenogeneic hepatocyte source for the clinical application of a BAL may present cellular and humoral immunological problems and the risk of PERV transmission. The first can be reduced to an acceptable proportion by using such a BAL not longer than 1 week. The second might be made quite acceptable by using viral reducing techniques in the circuit like UV treatment of the returning plasma or virus‐‐filters ((Nyberg et al., [Citation1999])).
The best solution of the above mentioned problems will be successful development of sufficient amounts of a well differentiated non‐‐tumorigenic human liver cell line.
Good bioreactors are available from the second millennium; the third millennium will give us the right liver cells!
References
- Ash S. R., Blake D. E., Carr D. J. Clinical effects of a sorbent suspension dialysis system in treatment of hepatic coma ((the Biologic DT)). Int. J. Artif. Organs 1992; 5(3)151–161
- Baquerizo A., Mhoyan A., Shirwan A. Xenobody response of patients with severe acute liver failure exposed to porcine antigens following treatment with a bioartificial liver. Trans. Proc. 1997; 29: 964–965
- Chamuleau R. A.F.M. Treatment of acute hepatic encephalopathy. Neth. J. Med. 1979; 22: 203–209
- Dixit G. G. The bioartificial liver: state of the art. Eur. J. Surg. 1998; 582(S)71–76
- Dixit V., Gitnick G. Transplantation of microencapsulated hepatocytes for liver function replacement. J. Biomater. Sci., Polym. Ed. 1995; 7(4)343–357
- Donini A., Baccarani U., Lavaroni S., Cautero N., Degrassi A. Temporary neurological improvement after bioartificial liver treatment for acute on chronic failure. ASAIO J. 2000; 46(2)241
- Ellis A. J., Hughes R. D., Wendon J. A., Dunne J., Langley P. G., Kelly J. H., Gislason G. T., Sussman N. L., Williams R. Pilot‐‐controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology 1996; 24: 1446–1451
- Flendrig L. M., Chamuleau R.A.F. M., Maas M.A. W. In vivo evaluation of a novel bioartificial liver in rats with complete liver ischemia: treatment efficacy and species‐‐specific alpha GST as a first attempt to monitor hepatocyte viability. J. Hepatol. 1999a; 30(2)311–320
- Flendrig L. M., Calise F., Di Florio E., Mancini M. Significant improvement of survival time in pigs with complete liver ischemia treated with a novel bioartificial liver. Int. J. Artif. Organs 1999b; 22(10)701–709
- Flendrig L. M., La Soe J. W., Jörning G. G., Steenbeek A., Karlsen O. T., Bovée W.M.M. J., Ladiges N.C.J. J., Velde A. A., Chamuleau R.A.F. M. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound non‐‐woven polyester matrix for hepatocyte culture as small aggregates. J. Hepatol. 1997; 26: 1379–1392
- Gerlach J. C. Development of a hybrid liver support system: a review. Int. J. Artif. Organs 1996; 19: 645–654
- Jauregui H. O., Mullon C.J. P., Trenkler D., Naik S., Santangini H., Press P., Muller T. E., Solomon B. A. In vivo evaluation of a hollow fiber liver assist device. Hepatology 1995; 21: 460–469
- Kardassis D., Busse B., Kraemer M. R., Smith M. D., Neuhaus P., Gerlach J. C. Hemodynamic effects of therapy with a hybrid liver support system. ASAIO J. 1999; 45(2)201
- Kelly J. H., Koussayer T., He D., Sussman N. L. Assessment of an extracorporeal liver assist device in anhepatic dogs. Artif. Organs 1992; 16: 418–423
- Kobayashi N., Fujiwara T., Westerman K., Inoue Y., Sakaguchi M., Noguchi H., Miyazaki M., Cai J., Tanaka N., Fox I. J., Leboulch P. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science 2000; 287: 1258–1262
- Kubota H., Reid L. M. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc. Natl. Acad. Sci. U. S. A. 2000; 97: 12132–12137
- Morimoto T., Matsushima M., Sowa N., Ide K., Sawanishi K. Plasma adsorption using bilirubin adsorbent materials as a treatment for patients with hepatic failure. Artif. Organs 1989; 13: 447–542
- Naruse K., Nagashima I., Sakai Y., Harihara Y., Jiang G. X., Suzuki M., Muto T., Makuuchi M. Efficacy of a bioreactor filled with porcine hepatocytes immobilized on nonwoven fabric for ex vivo direct hemoperfusion treatment of liver failure in pigs. Artif. Organs 1998; 22(12)1031–1037
- Nyberg S. L., Hibbs J. R., Hardin J. A., Germer J. J., Persing D. H. Transfer of porcine endogenous retroviruses across hollow fiber membranes. Transplantation 1999; 67(9)1251–1255
- Nyberg S. L., Shatford R. A. Evaluation of a hepatocyte‐‐entrapment hollow fiber bioreactor: a potential bioartificial liver. Biotechnol. Bioeng. 1993; 41: 194–203
- O'Grady J. G., Gimson A. E., O'Brien C. J. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology 1988; 94: 1186–1192
- Paradis K., Langford G., Long Z. Search for cross‐‐species transmission of porcine endogenous retrovirus in patients with living pig tissue. Science 1999; 285: 1236–1241
- Patience C., Takeuchi Y., Weiss R. B. Infection of human cells by an endogenous retrovirus of pigs. Nature Med. 1997; 3: 282–286
- Patzer J. F., Kramer D. J., Mazariegos G. V., Lopez‐‐Solis R. C., Giraldo M., Grogan T. A., Zhu Y., Fulmer M. L., Amiot B. P. Clinical safety evaluation of excorp medical, inc. Bioartificial liver support system ((BLSS)). ASAIO J. 2000; 46(2)241
- Pazzi P., Morsiani E., Muraca M., Vilei M. T., Rozga J., Demetriou A. A. Metabolic characterization of bioartificial liver: focus on bile production and excretion. Bioartificial Liver Support Systems. The Critical Issues, G. Crepaldi, A. A. Demetriou, M. Muraca. CIC Edizioni Internationali, Roma 1997; 42–47
- Riordan S. M., Williams R. Acute liver failure: targeted artificial and hepatocyte‐‐based support of liver regeneration and reversal of multiorgan failure [[Review]]. J. Hepatol. 2000; 32(S 1)63–76
- Rozga J., Holzman M. D., Ro M. S., Griffin D. W., Neuzil D. F., Giorgio T., Moscioni A. D., Demetriou A. A. Development of a hybrid bioartificial liver. Ann. Surg. 1993; 217: 502–515
- Sheil A. G.R., Sun J., Mears D. C., Waring M., Woodman K., Johnston B., Horvat M., Watson J., Kuotalistras N., Wang L. Positive biochemical effects of a bioartificial liver supprt system ((BALSS)) in a porcine fulminant hepatic failure ((FHF)) model. Int. J. Artif. Organs 1998; 21: 43–48
- Sarkis R., Wen L., Honiger J. Intraperitoneal transplantation of isolated hepatocytes of the pig: the implantable bioartificial liver. Chirurgie 1998; 123: 41–46
- Sielaff T. D., Hu M. Y., Amiot B., Rollins M. D., Rao S., McGuire B., Bloomer J. R., Hu W. S., Cerra F. B. Gel‐‐entrapment bioartificial liver therapy in galactosamine hepatitis. J. Surg. Res. 1995; 59: 179–184
- Stange J., Mitzner S. R., Risler T., Erley C. M., Lauchart W., Goehl H., Klammt S. Molecular adsorbent recycling system ((MARS)): clinical results of a new membrane‐‐based blood purification system for bioartificial liver support. Artif. Organs 1999; 23: 319–330
- Sussman N. L. Fulminant Hepatic Failure. Hepatology: A Textbook of Liver Disease3rd Ed., D. Zakim, T. D. Boyer. W.B. Saunders Company, Philadelphia, London, Sydney 1996; 618–650
- Sussman N. L., Gislason G. T., Kelly J. H. Extracorporeal liver support. Application to fulminant hepatic failure. J. Clin. Gastroenterol. 1994; 18(4)320–324
- Te Velde A. A., Flendrig L. M., Ladiges N.C.J. J., Chamuleau R.A.F. M. Possible immunological problems of bioartificial liver support. Int. J. Artif. Organs 1997; 20: 418–421
- Tygstrup N., Larson F., Hanson B. Treatment of acute liver failure by high volume plasmapheresis. In Acute Liver Failure, W. Lee, R. Williams. Cambridge university Press, Cambridge 1997; 267–277
- Uchino J., Tsuburya T., Kumagai F., Hase T., Hamada T., Komai T., Funatsu A., Hashimura K., Nakamura K., Kon T. A hybrid artificial liver composed of multiplated hepatocyte monolayers. ASAIO Trans. 1988; 34: 972–977
- Watanabe F. D., Mullon C.J. P., Hewitt W. R. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann. Surg. 1997; 225(5)484–494