Introduction
Hemodialysis insures the survival of more than 900,000 patients with chronic renal failure worldwide. Despite the advances in renal transplantation and organ procurement, the rate of increase of the dialytic population remains steady up to a 6%% yearly in the developed world. The picture is however incomplete still today. In developing countries, hemodialysis is still not a routine therapy and//or is still based on extensive, uncontrolled reuse. Nevertheless, even in countries where hemodialysis is an established routine practice, both mortality and morbidity are still very high. The major cause is cardiovascular disease which in recent years according to different National Registries has reached true epidemic proportions.
The development of new strategies in hemodialysis must meet today's patients' needs, be applicable in the context of the present cost pressure with the final goal of insuring clinically relevant advantages. In the past years, the Clinical and Laboratory Research Department at Bellco has followed several lines of research in order to develop new strategies for the removal of accumulated waste products in both acute and chronic renal failure as listed:
The concept of adsorption ((Hemodiafiltration with on‐‐line reinfusion of regenerated ultrafiltrate, HFR on line))
The reduction of oxidant stress, and the removal of protein‐‐bound, hydrophic “uremic toxins” ((hemolipodialysis, HLD))
The establishment of a dialysis system that incorporates the assurance of a “clean dialysis” machine and the on‐‐line production of dialysis fluid with an on‐‐line continuous check on fiber integrity. This technique was named as Paired HemodiaFiltration ((PHF))
In the present editorial, we will focus on two of these stategies namely the HFR on line, hemolipodialysis and the PHF (()).
Inherent to chronic uremia is the accumulation of metabolically active substances that may be responsible for the complex alterations of different biochemical pathways ((Vanholder et al., [Citation1996])). Hemodialysis is a physical treatment that removes water, electrolyte, urea and “uremic toxins” by diffusion, and//or convection. However, convection and diffusion, either alone or in combination, are largely unsatisfactory in removing “uremic toxins”. Adsorption is a third mechanism that has been applied in extracorporeal therapies. Early studies had shown that “uremic ((chromatographic)) peaks” can be efficiently eliminated in haemofiltration with on‐‐line regeneration of the ultrafiltrate, resulting in increased hematocrit and reducing β2‐‐microglobulin levels ((Cerulli et al., [Citation1987])).
In haemodiafiltration ((HDF)), the use of sorbents could be possible using the double chamber dialyser in Paired Filtration Dialysis ((PFD))))((Ghezzi et al., [Citation1983], [Citation1992])) and the first clinical trial be performed ((De Francisco et al., [Citation1997])).
Originally, the concept of adsorption was applied to the regeneration of dialysate using charcoal‐‐based sorbents in the search for an easily movable artificial kidney ((Dunea and Kolff, [Citation1965])). Subsequently, Shaldon et al. (([Citation1978])) proposed the regeneration of the dialysate via perfusion through the Redy Sorbent Cartridge. However, the need to add aluminum oxide or other alumina salts to insure stabilization of the urease as well as easy releasibility of microaggregates led to high levels of aluminum with cases of overt aluminum intoxication ((Pierides and Frohnert, [Citation1981])). The concept of fluid regeneration has been recently revisited in the haemodiabsorption technique where a charcoal‐‐based sorbent mixed with dialysate is recirculated in a closed dialysate circuit ((Ash, [Citation1994])).
A well‐‐known field for the application of a sorbent has been in the therapy of both chronic renal failure ((haemoperfusion in combination with haemodialysis)) or in the treatment of acute poisoning ((Yatzidis, [Citation1971]; Yatzidis et al., [Citation1976])). At the time of both benzodiazepine and salicylate accidental or voluntary poisoning, charcoal offered a useful tool for adsorbing the substances directly from the blood stream because of its high adsorbing capacity and high available surface. However, in extracorporeal therapies, the remarkable characteristics of charcoal for adsorbing a wide range of compounds have been always confronted with the high degree of bioincompatibility. The charcoal in the “coated” form acquires a much better compatibility in whole blood but greatly diminishes its adsorptive capacities.
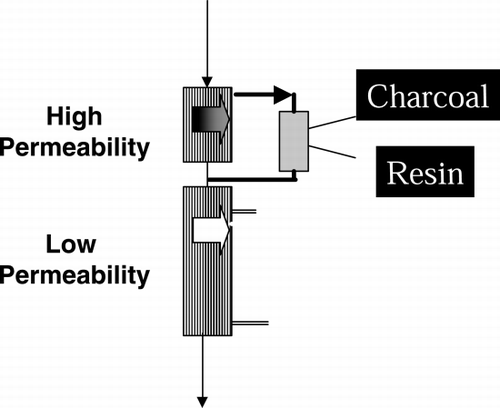
As it was stated above, the double chamber technique ((also named as Paired Filtration Dialysis, PFD)) is the only existing hemodiafiltration ((HDF)) technique that allows on‐‐line regeneration of the ultrafiltrate. This property relies on the physical separation of convection from diffusion. Pure ultrafiltrate ((patient's own plasma water)) can be passed through the sorbent cartridge. On‐‐line regeneration of the ultrafiltrate brings up another important advantage in that sorbents may be used in the “uncoated” form, a remarkable advantage over the “coated” one that is mandatory when sorbents be used in whole blood. More recently, beside charcoal, synthetic sorbents have become available in different extracorporeal therapies: stationary matrixes now encompass ion‐‐exchange ((Ash, [Citation1994])), or hydrophobic resins ((Tetta et al., [Citation1998])). Recently, a flow distribution study using standard radiopharmaceutical hydroxyethylenediphosphonate, labelled with technetium‐‐99m as marker of the saline flow through the column has allowed the imaging of molecule distribution with a γ camera during the various phases of the radiolabelled molecule transit in the cartridge. These studies have shown the impressive homogeneity in distribution of the tracer during the passage of aqueous solution such as the ultrafiltrate.
The second chamber of PFD is made of a low permeability membrane to insure diffusion and patient's weight loss. The low permeability diffusive membrane eliminates the risk of backfiltration. Backfiltration of dialysate occurs using high permeable membranes in high‐‐flux haemodialysis and HDF ((Ronco et al., [Citation1992])). The transmembrane passage of bacterial pyrogens contaminating the dialysate may lead to monocyte activation, and cytokine production, thus representing a mechanism capable of triggering systemic inflammation [[reviewed in Botella et al., [Citation2000]]]. To avoid such low level of biocompatibility, two approaches have been suggested and proved to be effective: the use of ultrapure dialysate and the adoption of techniques that abrogate backfiltration.
A recent, long‐‐term, prospective, cross‐‐over study in patients on PFD showed a significant reduction in the generation of both interleukin‐‐1 ((IL‐‐1)) and IL‐‐1 receptor antagonist ((IL‐‐1Ra)) in patients treated with PFD versus patients on conventional HDF ((Panichi et al., [Citation1998a])). The reduced cytokine production in PFD has been related to the absence of backfiltration, a major driving force in the stimulation of monocytes from contaminated dialysate ((Schindler et al., [Citation1997])). The same multicenter study ((Panichi et al., [Citation1998a])) also showed significantly higher plasma levels of C‐‐reactive protein and IL‐‐6 in patients on conventional HDF than in PFD ((Panichi et al., [Citation1998b])). In a prospective, long‐‐term clinical survey on 302 haemodialysed patients, the highest values of C‐‐reactive protein and IL‐‐6 were shown in patients on conventional HDF, but not in PFD ((Panichi et al., [Citation2000])). In addition, reinfusion, albeit partial, of hydrosoluble vitamins and aminoacids, as it occurs in HFR on‐‐line ((unpublished results)) could theoretically ameliorate the nutritional status. Hydrophilic vitamins such as vitamin C are lost during HDF ((J.P. Cristol, B.Canaud, personal communication)). This loss may further worsen the oxidant//antioxidant unbalance that is known to occur in chronically uremic patients in haemodialysis ((Cristol et al., [Citation1994])). Haemodialysis may trigger the formation of radical oxygen species in bioincompatibility‐‐associated phenomena: namely O2°‐‐, its metabolites ((H2O2 and .OH)) and hydroperoxides released from arachidonic acid ((Tetta et al., [Citation1999])). Several antioxidant defenses are altered in hemodialysis patients as exemplified by a reduction in the activity of Cu//Zn superoxide dismutase in erythrocytes and glutathione peroxidase ((Takahashi et al., [Citation1986])). Finally, the depletion of vitamin C further aggravates the state of enhanced oxidation of vitamin E ((Sullivan and Eisenstein, [Citation1970]; Descombes et al., [Citation1993])). A Multicenter European Collaborative Study is presently ongoing as it has been recently published concerning methodology and its main outcomes ((Cerulli et al., [Citation2000])).
Hemolipodialysis
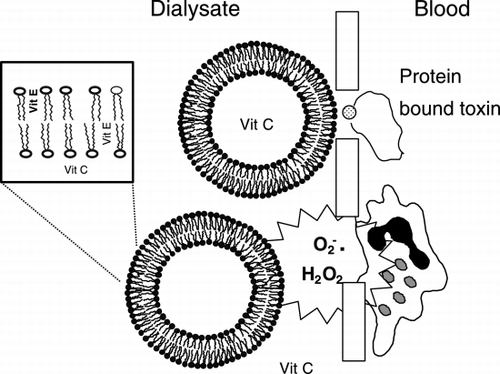
Hemodialysis is associated with increased oxidative stress and poor removal of certain hydrophobic and protein bound toxins ((Tetta et al., [Citation1999])). Hemolipodialysis ((HLD)), exploits the amphipathic nature of liposomes added to the dialysate to increase removal of hydrophobic toxins and to function together with α‐‐tocopherol ((vitamin E)) and ascorbic acid ((vitamin C)) to reduce oxidant stress. This may be helpful in hemodialysis as most toxins during conventional dialysis are removed by diffusion, convection or membrane adsorption. Hemolipodialysis increases the removal efficiency of uremic toxins while reducing oxidant stress associated with extracorporeal therapies ((Wratten et al., [Citation1999])) (()).
The liposomes used in HLD are approximately 250–300 nm in diameter. They interact at the asymmetric interface of polyethersulfone membrane pores without actual passage through the membrane. The liposomes are comprised of the unsaturated phospholipid, soybean phosphatidylcholine in a lyophilized form, which spontaneously form a unilamellar bilayer upon addition of physiological buffer or dialysate. Vitamin E is incorporated into the liposome bilayer ((and thus doesn't actually pass into the patients' blood)), while vitamin C is added to the acid concentrate of the dialysate. The acidic nature of the concentrate helps maintain vitamin C in a reduced ((active form)) ((Wratten et al., [Citation1999])).
Vitamin C and Vitamin E are used together to synergistically support the patient's antioxidant defense system. Ascorbic acid can recycle α‐‐tocopherol from the tocopheroxyl free radical, thus maintaining vitamin E in its active form ((reviewed in Tetta et al., [Citation1999])).
While healthy individuals have a well‐‐maintained balance of antioxidants to counter oxidant stress, chronic hemodialysis patients appear to have inappropriate free radical production and an inadequate antioxidant defense system. Oxidative stress frequently occurs in hemodialysis patients due to net loss of water soluble antioxidants such as vitamin C and uric acid or inflammatory cell activation due to bio‐‐incompatible membranes or dialysate containing cytokine‐‐inducing substances ((Morena et al., [Citation2000])). Oxidative stress has been suggested to play a role in many of the complications associated with chronic renal failure and hemodialysis such as cardiovascular complications and amyloidosis ((Wratten et al., [Citation2000a])).
Free radicals can damage proteins, lipids, DNA, sugars, as well as biological metabolites such as creatinine. We have recently reported in vitro evidence that suggests that dialysate containing liposomes and antioxidants may be beneficial in preventing oxidant stress as measured by changes in the oxidation of lipid and protein components, and by the removal of hydrophobic substances, such as bilirubin and platelet activating factor ((PAF)) that may participate in inflammatory reactions ((Wratten et al., [Citation2000b])).
Convective therapies have been shown to improve treatment tolerance and enhance middle molecule removal when compared to standard hemodialysis. However the high cost of these procedures is a limiting factor for their widespread applicability in any chronic dialysis setting, particularly in this era of limited health care funding. Technogical advances now permit on‐‐line production of substitution fluid, thereby significantly reducing the costs associated with convective treatments.
Several clinical studies published so far assessed optimal clinical outcomes and safety of on‐‐line procedures in the long term run ((Canaud et al., [Citation2000a], Citation[b]; Altieri et al., [Citation1997]; Maduell et al., [Citation1999])). Recently, Pizzarelli and Maggiore (([Citation1998])) and Pizzarelli et al. (([Citation1998])) published data showing on‐‐line fluids have the same, possibly less, cytokine inducing capability than commercially produced reinfusion fluids. Some monitors specifically conceived for on‐‐line convective treatments have been recently introduced in the market. They fully comply with the regulations for medical devices. Sterility and apirogenicity of infusion fluid is obtained by cold filtration of dialysate and is guarantee by pre‐‐treatment assessment of ultrafilter fiber integrity by pressure test and//or redundancy of dialysate ultrafilters. Notwithstanding the reassuring evidence, many nephrologists are still concerned about the impossibility to check in real time the sterility and apirogenicity of the on‐‐line produced reinfusion fluid.
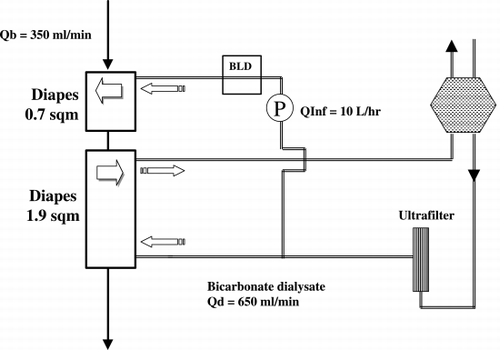
Pizzarelli et al. (([Citation2000])) presented clinical and biological data of PHF, a new on‐‐line treatment. This technique relied on the backfiltration of dialysate and was performed with the a double chamber hemodialyzer (()). The peculiar geometry of this dialyser, consisting of two chambers connected in series, allowed on‐‐line monitoring of the integrity of the dialysate ultrafilter fibers during treatment. By so doing, this device could offset, or at least mitigate, concerns about safety of on‐‐line treatments.
References
- Altieri P., Sorba G. B., Bolasco P. G., Bostrom M., Asproni E., Ferrara R. On‐‐line predilution hemofiltration versus ultrapure high‐‐flux hemodialysis: a multicenter prospective study in 23 patients. Blood Purif. 1997; 15: 169–181
- Ash S. R. Hemodiabsorption in treatment of acute hepatic failure and chronic cirrhosis with ascites. Artif. Organs 1994; 18(5)355–362
- Chronic Inflammation in Haemodialysis, J. Botella, G. La Greca, H. Klinkmann, P. Zucchelli. Karger, Basel 2000
- Canaud B., Bosc J. Y., Leray‐‐Moragues H., Stef F., Argiles A., Leblanc M., Mion C. On‐‐line haemodiafiltration: safety and efficacy in long‐‐term clinical practice. Nephrol. Dial. Transplant. 2000a; 15(1S)60–67
- Canaud B., Bosc J. Y., Leray‐‐Moragues H., Morena M., Stec F. Microbiologic purity of dialysate: rationale and technical aspects. Blood Purif. 2000b; 18(3)200–213
- Cerulli N., Politi L., D'Angelo A. R., Scandurra R. A purification method by uncoated charcoal. Adv. Exp. Med. Biol. 1987; 223: 193–196
- Cerulli N., La Greca G., Laville M., et al. The effect of hemodiafiltration with on‐‐line endogenous reinfusion ((on‐‐line HFR)) on anemia: design of a European, open, randomised, multicentre trial. J. Nephrol. 2000; 13: 34–42
- Cristol J. P., Canaud B., Rabesandratana H., et al. Enhancement of reactive oxygen species production and cell surface markers expression during hemodialysis. Nephrol. Dial. Transplant. 1994; 9: 389–394
- De Francisco A.L. M., Botella J., Escallada R., et al. Hemodiafiltration with sorbent‐‐regenerated ultrafiltrate as replacement fluid: a multicenter study. Nephrol. Dial. Transplant. 1997; 12(3)528–534
- Descombes E., Hanck A. B., Fellay G. Water soluble vitamins in chronic hemodialysis patients and need for supplementation. Kidney Int. 1993; 43: 19–28
- Dunea G., Kolff W. J. Clinical experience with the Yatzidis charcoal artificial kidney. Trans. Am. Soc. Artif. Intern. Organs 1965; 2: 178–182
- Ghezzi P. M., Frigato G., Fantini G. F., et al. Theoretical model and first clinical results of paired filtration dialysis. Life Support Syst. 1983; 1(S)271–275
- Ghezzi P. M., Gervasio R., Tessore V., et al. Hemodiafiltration without replacement fluid. ASAIO J. 1992; 38(1)61–65
- Maduell F., del Pozo C., Garzia H., Sanchez L., Hdez‐‐Iaras J., Alber M. D., Calvo C., Torregrosa I., Navarro V. Change from conventional haemodiafiltration to on‐‐line haemodiafiltration. Nephrol. Dial. Transplant. 1999; 14: 1202–1207
- Morena M., Cristol J. P., Canaud B. Why hemodialysis patients are in a pro‐‐oxidant state? What could be done to correct the pro//antioxidant imbalance?. Blood Purif. 2000; 18(3)191–199
- Panichi V., De Pietro S., Andreini B., Migliori M., Tessore V., Taccola D., Rindi P., Palla R., Tetta C. Cytokine production in haemodiafiltration: a multicentre study. Nephrol. Dial. Transplant. 1998a; 13(7)1737–1744
- Panichi V., Tetta C., Rindi P., Palla R., Lonnemann G. Plasma C‐‐reactive protein is linked to backfiltration associated interleukin‐‐6 production. ASAIO J. 1998b; 44(5)M415–M417
- Panichi V., Migliori M., De Pietro S., Metelli M. P., Taccola D., Perez R., Palla R., Rindi P., Cristofani R., Tetta C. Plasma C‐‐reactive protein in hemodialysis patients: a cross‐‐sectional, longitudinal clinical survey. Blood Purif. 2000; 18(1)30–36
- Pierides A. M., Frohnert P. P. Aluminum related dialysis osteomalacia and dementia after prolonged use of the Redy cartridge ((Abstr)). ASAIO 1981; 10: 53
- Pizzarelli F., Maggiore Q. Clinical perspectives of on‐‐line haemodiafiltration. Nephrol. Dial. Transplant. 1998; 13(S5)34–37
- Pizzarelli F., Cerrai T., Dattolo P., Tetta C., Maggiore Q. Convective treatments with on‐‐line production of replacement fluid: a clinical experience lasting 6 years. Nephrol. Dial. Transplant. 1998; 13: 363–369
- Pizzarelli F., Tetta C., Cerrai T., Maggiore Q. Double chamber on‐‐line hemodiafiltration: a novel technique with intra‐‐treatment monitoring of dialysate ultrafilter integrity. Blood Purif. 2000; 15(3)237–241
- Ronco C., Brendolan A., Feriani M., Milan M., Conz P., Lupi A., Berto P., Bettini M., La Greca G. A new scintigraphic method to characterize ultrafiltration in hollow fiber dialyzers. Kidney Int. 1992; 41(5)1383–1393
- Schindler R., Serovka F., Frei U. Transport mechanisms of cytokine‐‐inducing substances through hemodialyzers ((Abstract A1159)). J. Am. Soc. Nephrol. 1997; 8: 252A
- Shaldon S., Beau M. C., Carlet G., et al. Sorbent regeneration of ultrafiltrate as long term treatment of end stage renal failure. Dial. Transplant. 1978; 708–711
- Sullivan J. F., Eisenstein A. B. Ascorbic acid depletion in patients undergoing hemodialysis. Am. J. Clin. Nutr. 1970; 23: 1339–1346
- Takahashi K., Newburger P. E., Cohen H. J. Glutathione peroxidase protein absence in selenium deficiency and correlation with enzymatic activity. J. Clin. Invest. 1986; 77: 1402–1404
- Tetta C., Cavaillon J. M., Schultze M., Ronco C., Ghezzi P. M., Camussi G., Serra A. M., Curti F., Lonnemann G. Removal of cytokines and activated complement components in an experimental model of continuous plasmafiltration coupled with sorbent adsorption. Nephrol. Dial. Transplant. 1998; 13: 1458–1464
- Tetta C., Biasioli S., Schiavon R., Inguaggiato P., David S., Panichi V., Wratten M. L. An overview of hemodialysis and oxidative stress. Blood Purif. 1999; 17(2–3)64–72
- Vanholder R., De Smet R., Vogeleere P., . The uraemic syndrome. Replacement of Renal Function by Dialysis4th Ed., C. Jacobs, C. M. Kjellstrand, K. M. Koch, J. F. Winchester, et al. Kluwer Academic Publishers. 1996; 1–33
- Wratten M. L., Navino C., Tetta C., Verzetti G. Haemolipodialysis. Blood Purif. 1999; 17(2–3)127–133
- Wratten M. L., Tetta C., Ursini F., Sevanian A. Oxidant stress in hemodialysis: prevention and treatment strategies. Kidney Int. 2000a; 58(76S)126–132
- Wratten M. L., Sereni L., Tetta C. Hemolipodialysis attenuates oxidative stress and removes hydrophobic toxins. Artif. Organs 2000b; 24(9)685–690
- Yatzidis H. The use of ion exchange resins and charcoal in acute barbiturate poisoning. Acute Barbiturate Poisoning, H. Matthew. Excerpta Medica, Amsterdam 1971; 223–232
- Yatzidis H., Yulis G., Digenis P. Hemocarboperfusion‐‐hemodialysis treatment in terminal renal failure. Kidney Int. 1976; 10(S)312–314