Abstract
In hemodialysis patients, oxidative stress results from an imbalance between the production of reactive oxygen species and antioxidant defense mechanisms. Recently, a new dialysis multi‐‐layer membrane has been developed, by modifying the inner surface of regenerated cellulose to support a vitamin E coating.
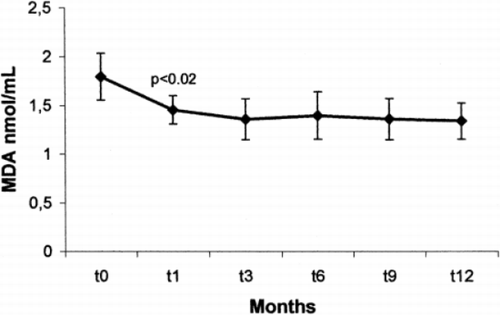
The aim of our study was to investigate the effects of hemodialysis treatment with vitamin E‐‐modified membrane on anemia and erythropoietin requirement in a group of chronic uremic patients. Ten uremic, non diabetic, patients on standard bicarbonate dialysis were treated with vitamin E‐‐bonded dialysis membrane for 12 months. Hematological parameters, erythropoietin requirement, serum vitamin E and serum malonyldialdehyde ((MDA)) were evaluated before starting the study and monthly. No significant changes in hemoglobin level, RBC count, hematocrit and EPO requirement were observed. Basal vitamin E levels were in the normal range ((13.0±2.88 mg//L vs. 14.79±3.12 mg//L; NS)). On the contrary, basal MDA levels were higher than those observed in the control group ((1.87±0.36 vs. 1.13±0.18 mmol//mL; p < 0.01)) and a significant decrease of MDA levels was found after 1 month of Excebrane® treatment ((1.39±0.25 nmol//mL; p < 0.02)).
In conclusion, the role of the “oxidative hemolysis” in the pathogenesis of anemia in CHD patients is still not clearly defined, but it could be of minor clinical relevance. Although the effectiveness of vitamin E‐‐coated membranes as a scavenger of ROS allows a better control of intradialytic oxidative stress, it doesn't seem to contribute to clinical management of anemia in these patients.
Introduction
Oxidative stress which results from an imbalance between the production of reactive oxygen species ((ROS)) and antioxidant defense mechanisms is well recognized in chronic hemodialysis ((CHD)) patients ((Galli et al., [Citation1999]; Hasselwander and Young, [Citation1998]; Lougherey et al., [Citation1994]; Morena et al., [Citation2000])) and could be involved in uremic‐‐related pathologies, such as immunological and coagulative dysfunctions, cataract, amyloidosis and atherosclerosis ((Lougherey et al., [Citation1994]; Magi et al., [Citation1994]; Morena et al., [Citation2000])). The increased production of ROS during the dialytic session, due to leukocyte oxidative metabolism activation, contributes to red blood cells ((RBC)) lipid peroxidation and haemolysis and could play a role in the pathogenesis of anemia in CHD patients ((Taccone‐‐Gallucci et al., [Citation1999])).
Vitamin E is considered the most important antioxidant agent of the cellular membranes ((Taccone‐‐Gallucci et al., [Citation1999])). In uremic patients, plasma levels of vitamin E were reported both normal or slightly reduced ((Triolo et al., [Citation1994]; Zima et al., [Citation1998])). Recently, a new dialysis multi‐‐layer membrane has been developed, by modifying the inner surface of regenerated cellulose to support a vitamin E coating ((Excebrane®, Terumo Corporation, Japan)) ((Sasaki et al., [Citation1999])). Some studies ((Galli et al., [Citation1998]; Tarng et al., [Citation2000])) report that Excebrane® filters can be effective in reducing ROS‐‐induced damage in CHD patients, thus providing a better control of lipoperoxidation.
In this study, we investigated the effects of CHD treatment with vitamin E‐‐modified membrane on anemia and erythropoietin requirement, and the changes of oxidative status in a group of chronic uremic patients.
Patients and Methods
Ten uremic, non diabetic, patients ((6 male and 4 female; mean age: 64.6±13.7 years; dialytic age: 86.6±47.2 months)) were selected for the study. Primary renal disease was nephroangiosclerosis in 5 cases, glomerulonephritis in 3 patients, interstitial nephritis in 2 cases. All patients had been on standard bicarbonate dialysis ((3.5–4.0 h, 3 times a week)) with synthetic membrane before starting a period of treatment with Excebrane® filters, Clirans®E 15–18 NL, for 12 months.
RBC count, hemoglobin levels, hematocrit, transferrin saturation, serum ferritin, EPO requirement, Kt//V, serum vitamin E and serum malonyldialdehyde ((MDA)) ((an intermediate product of lipid peroxidation)) were evaluated before starting the study and monthly. Blood samples were collected just before the dialytic session. Serum samples used for vitamin E and MDA determinations were frozen at − 20°C and processed within 3 days. Vitamin E was measured by high‐‐performance liquid chromatography ((HPLC)) as previously reported ((Triolo et al., [Citation1994])). MDA was detected by HPLC according to Wong et al. (([Citation1987])). Thirty healthy subjects were randomly selected as control group ((17 M, 13 F; mean age 59.8±9.4 years)).
During the 12 months of the study, iron supplementation ((intravenous ferrous gluconate)) was provided to four patients to maintain iron stores in the normal range ((serum ferritin > 100 ng//mL, transferritin saturation > 20%%)).
Results are reported as mean±SD. Statistical evaluations were performed by paired Student's t test; p < 0.05 was considered as significant.
Results
shows the data obtained at the basal time ((t0)) and after three ((t3)), six ((t6)) and twelve ((t12)) months. No significant changes in hemoglobin level, RBC count, hematocrit and EPO requirement were observed. Serum ferritin, tranferrin saturation and Kt//V were maintained stable all over the duration of study. As reported in , vitamin E levels were in the normal range ((13.01±2.88 mg//L vs. 14.79±3.12 mg//L; NS)) before starting dialysis treatment with Excebrane® and showed a slight, not significant increasing trend during the 12 months ((15.88±3.92 mg//L; NS)). On the contrary, basal MDA levels were higher than those observed in the control group ((1.87±0.36 vs. 1.13±0.18 nmol//mL; p < 0.01)). Moreover, a significant decrease of MDA levels was found after 1 month of Excebrane® treatment ((1.39±0.25 nmol//mL; p < 0.02)) (()) and were maintained throughout the study ((t3: 1.31±0.22; t6: 1.27±0.24; t12: 1.29±0.18 nmol//mL)).
Table 1. Hematological and biochemical parameters before and during dialisys treatment with vitamin E‐‐modified membrane
Table 2. Vitamin E and MDA levels before and during dialisys treatment with vitamin E‐‐modified membrane
Discussion
Chronic renal failure is associated with a condition of oxidative stress, which results from accumulation of toxins with prooxidant properties and from a reduced activity of protective systems related to increased consumption or decreased dietary intake of antioxidants. In addition, low biocompatibility of hemodialysis procedure implies an activation of leukocyte oxidative metabolism with ROS generation and oxidation of proteins, sugars and polyunsaturated lipids ((Galli et al., [Citation1999]; Hasselwander and Young, [Citation1998]; Lougherey et al., [Citation1994]; Morena et al., [Citation2000]; Triolo et al., [Citation1994])). The increased lipoperoxidation of cellular membrane is related to a reduced activity of pentose–phosphate shunt and contributes to chronic damage of erythrocyte membrane and hemolysis ((Yawata and Jacob, [Citation1975])). In CHD patients, increased levels of RBC MDA and a reduction of RBC vitamin E were described ((Giardini et al., [Citation1984a])). The role of vitamin E supplementation to correct the effects of oxidant stress is still controversial. Giardini et al. (([Citation1984a], Citation[b])) showed a significant reduction of oxidative damage of RBC membrane, an increase of hematocrit and a significant reduction of chronic hemolysis in CHD patients treated with parenteral vitamin E. Although some studies ((Ono, [Citation1985]; Cristol et al., [Citation1997])) have reported similar results, other Authors have not confirmed these data ((Aguilera et al., [Citation1993]; Lillo‐‐Ferez et al., [Citation1987])).
Vitamin E‐‐modified dialysis filters have demonstrated an effective scavenging function against ROS, during hemodialysis session ((Galli et al., [Citation1998])). A significant increase in RBC count, hemoglobin and hematocrit has been reported after 6 months on Excebrane® dialysis treatment, associated with an improvement of RBC survival ((Takemoto et al., [Citation2001])). A reduction of chronic hemolysis has been shown ((Taccone‐‐Gallucci et al., [Citation1999])) in 10 CHD patients in which hemoglobin levels remained stable during one year of dialysis treatment with Clirans® E, in spite of EPO therapy discontinuation. In our study, neither significant variations of hematological parameters, nor modifications of EPO requirements were observed after 12 months of treatment with Excebrane®; nevertheless, our data confirm the beneficial effects of this membrane on lipid peroxidation, as evidenced by the persistent reduction on serum MDA.
The role of the “oxidative hemolysis” in the pathogenesis of anemia in CHD patients is still not clearly defined ((Eckardt, [Citation2000])), but it could be of minor clinical relevance. Although the effectiveness of vitamin E‐‐coated membranes as a scavenger of ROS allows a better control of intradialytic oxidative stress, it doesn't seem to contribute to clinical management of anemia in these patients.
In conclusion, our study confirmed the protective role of vitamin E‐‐modified membrane against dialytic oxidative stress and lipoperoxidation, but it didn't evidence any significant clinical effect on anemia, probably because of a minor role of oxidative hemolysis in the pathogenesis of uremic anemia.
References
- Aguilera A., Teruel J. L., Villatruela J. J., Rivera M., Ortuno J. Effect of vitamin E administration on erythropoietin values and anaemia in hemodialysis patients. Nephrol. Dial. Transplant. 1993; 8: 379
- Cristol J. P., Bosc J. Y., Leblanc M., Lorrho R., Descomps B., Canaud B. Erythropoietin and oxidative stress in haemodialysis: beneficial effects of vitamin E supplementation. Nephrol. Dial. Transplant. 1997; 12: 2312–2317
- Eckardt K. U. Pathophysiology of renal anemia. Clin. Nephrol. 2000; 1(S)S2–S8
- Galli F., Rovidati S., Chiarantini L., Campus G., Canestrari F., Buoncristiani U. Bioreactivity and biocompatibility of a vitamin E‐‐modified multi‐‐layer hemodialysis filter. Kidney Int. 1998; 54: 580–589
- Galli F., Canestrari F., Bellomo G. Pathophysiology of the oxidative stress and its implication in uremia and dialysis. Contrib. Nephrol. 1999; 127: 1–31
- Giardini O., Taccone‐‐Gallucci M., Lubrano R., Ricciardi‐‐Tenore G., Bandino D., Silvi I., Ruberto U., Casciani C. U. Evidence of red blood cell membrane lipid peroxidation in haemodialysis patients. Nephron 1984a; 36: 235–237
- Giardini O., Taccone‐‐Gallucci M., Lubrano R., Ricciardi‐‐Tenore G., Bandino D., Silvi I., Ruberto U., Paradisi C., Mannarino O., Citti G., Elli M., Casciani C. U. Effects of alphatocopherol administration on red blood cell membrane lipid peroxidation in hemodialysis patients. Clin. Nephrol. 1984b; 21: 174–177
- Hasselwander O., Young I. S. Oxidative stress in chronic renal failure. Free Radic. Res. 1998; 29: 1–11
- Lillo‐‐Ferez M., Allain B., Dupommereulle C., Prieur P., Petrover M. Inefficacy of vitamin E supplementation on anemia in hemodialysis patients. Nephron 1987; 45: 79–80
- Lougherey C. M., Young I. S., Lightbody J. H., McMaster D., McNamee P. T., Trimble E. R. Oxidative stress in hemodialysis. Q. J. Med. 1994; 87: 679–683
- Magi E., Bellazzi R., Falaschi F., Frattoni A., Perani G., Finardi G., Gazo A., Nai M., Romanini D., Bellomo G. Enhanced LDL oxidation in uremic patients: an additional mechanism for accelerated atherosclerosis?. Kidney Int. 1994; 45: 876–883
- Morena M., Cristol J. P., Canaud B. Why hemodialysis patients are in a prooxidant state? What could be done to correct the pro//antioxidant imbalance. Blood Purif. 2000; 18: 191–199
- Ono K. Effect of large dose vitamin E supplementation on anemia in hemodialysis patients. Nephron 1985; 40: 440–445
- Sasaki M., Hosoya N., Saruhashi M. Development of vitamin E‐‐modified membrane. Contrib. Nephrol. 1999; 127: 49–70
- Taccone‐‐Gallucci M., Lubrano R., Meloni C. Vitamin E as an antioxidant agent. Contrib. Nephrol. 1999; 127: 32–43
- Taccone‐‐Gallucci M., Meloni C., Lubrano R., Morosetti M., Palombo G., Cianciulli P., Scoppi P., Castello M. A., Casciani C. U. Chronic haemolysis and erythrocyte survival in haemodialysis patients treated with vitamin E‐‐modified dialysis filters. Contrib. Nephrol. 1999; 127: 44–48
- Takemoto Y., Tsuchida K., Nakatani T., Yamamoto K., Kishimoto T. (2001) The effect of vitamin E‐‐bonded dialyzer membrane on red blood cell survival in hemodialyzed patients. Blood Purification. Vol. 19. Proceedings of the 18th Annual Meeting of International Society of Blood Purification, Rome, 2000
- Tarng D. C., Huang T. P., Liu T. Y., Chen H. W., Sung Y. J., Wei Y. H. Effect of vitamin E‐‐bonded membrane on the 8‐‐hydroxy 2′‐‐deoxyguanosine level in leukocyte DNA of hemodialysis patients. Kidney Int. 2000; 58: 790–799
- Triolo L., Lippa S., Oradei A., De Sole P., Mori R. Serum coenzyme Q10 in uremic patients on chronic hemodialysis. Nephron 1994; 66: 170–175
- Wong S. H., Knight J. A., Hopfer S. M., Zaharia O., Leach C. N., Jr., Sunderman F. W., Jr. Lipoperoxides in plasma as measured by liquid‐‐chromatographic separation of malondialdehydethiobarbituric acid adduct. Clin. Chem. 1987; 33: 214–220
- Yawata Y., Jacob H. Abnormal red cell metabolism in patients with chronic uremia: nature of the defect and its persistence despite adequate hemodialysis. Blood 1975; 45: 231–239
- Zima T., Janebova M., Nemecek K., Bartova V. Retinol and alpha‐‐tocopherol in hemodialysis patients. Ren. Fail. 1998; 20: 505–512