Abstract
We have recently reported our study on novel nano‐dimension red blood cell (rbc) substitute based on ultrathin PEG‐PLA membrane nanocapsules (80–150 nanometer diameter) containing hemoglobin (Hb) and enzymes. These have a markedly increased the circulation half‐times as compared to our earlier PLA membrane nanocapsules. In the present study to be reported here, instead of looking at this from a pharmacodynamic point of view, we design the Hb nanocapsules from the point of view of transfusion medicine. For instance, the maximal levels of systemic non‐red blood cell (rbc) Hb that can be attained after one infusion of 30% blood volume of 10 gm/dl Hb in the form of different types of PEG‐PLA Hb nanocapsules or polyHb. Also the length of time one infusion can maintain a given systemic non‐rbc hemoglobin Hb level. Of the two types of polyhemoglobins similar to those in clinical trials but prepared in this laboratory, the maximal levels of Hb reached were 3.35 gm/dl and 3.10 gm/dl respectively. The times for the hemoglobin level to fall to 1.67 gm/dl were 14 hours and 10. hours respectively, corresponding to 24 hours and 17 hours in human. The best PEG‐PLA Hb nanocapsules are prepared using a combination of the following 4 factors: use of polymerized Hb, the use of higher M.W. PLA, the use of higher concentrations of PEG‐PLA and the crosslinking of the newly formed PEG‐PLA Hb nanocapsules. With this, the maximal non‐rbc systemic Hb reached was 3.66 gm/dl and the time to reach 1.67 gm/dl was 24.2 hours, or 41.5 hours in human if extrapolated using the results obtained with polyHb in rats.
Introduction
PolyHb is at present one of the most promising first generation blood substitutes (Chang, [Citation2002]; Chang et al., Citation[2002]; Gould et al., [Citation1998]; Sprung et al., [Citation2002]; Squires, [Citation2002]). Each polyhemoglobin consists of 3 to 6 hemoglobin molecules covalently crosslinked together to form a soluble macromolecule. Polyhemoglobins (polyHbs) are useful in many clinical applications, especially for perioperative uses in surgery. For other clinical applications, polyHb does not contain the needed antioxidant enzymes and can cause adverse effects in sustained hemorrhagic shock, stroke and other conditions (Alayash, [Citation1999]; Chang, [Citation2002]; D'Agnillo and Chang, [Citation1998]). Furthermore, the circulation half time after 30% blood volume infusion is only 12 hours in rats and about 24 hours in human.
We have prepared artificial red blood cells earlier based on microencapsulation of hemoglobin and all the red blood cell enzymes (Chang, [Citation1964]) including biodegradable polylactic acid membrane Hb microcapsules (Chang, [Citation1976]). However, these microcapsules only circulate for 30 minutes. More recently, we used a biodegradable polymer, polylactic acid to form much smaller nanocapsules (80–180 nanometer) each containing hemoglobin and red blood cell enzymes (Chang, [Citation1997]; Chang and Yu, Citation[1997]). However, these are removed within 2 hours after infusion. We have recently reported our study on novel methods for the preparation of nano‐dimension red blood cell (rbc) substitute.based on ultrathin PEG‐PLA membrane nanocapsules (80–150 nanometer diameter) containing Hb and enzymes (Chang and Yu, Citation[2001]; Citation[[Yu and Chang, submitted]). This resulted in marked increase in circulation half‐times from the original 2 hours to about 12 hours in rats (Chang and Yu, Citation[2001]; Citation[[Yu and Chang, submitted]). The research to be reported in the present paper resulted in further increase to double this (Chang et al., Citation[2002]). This includes an analysis of different types of PEG‐PLA Hb nanocapsules in the maintenance of systemic Hb levels as compared to polyHb. Instead of looking at this from a pharmacodynamic point of view (the rate of decrease and the extrapolation to zero time), the present analysis is to look at it from the point of view of transfusion medicine with two factors. 1) What are the maximal levels of systemic Hb that can be attained after one infusion of 1/3 blood volume of 10 gm/dl Hb in the form of different types of PEG‐PLA Hb nanocapsules or polyHb. 2) How long can one infusion maintain a given level of non‐rbc systemic Hb level?
Methods
(i) Preparation of the new PEG‐PLA copolymer: One and a half grams of DL‐PLA[M.W. 5,000 or 15,000] and 0.75 g of methoxypolyethylene glycol [M.W. 2000] were dried under vacuums overnight. Five ml of acetone was added. The mixture was heated to 180°C for 2 hr. under nitrogen. After adding 10 µl of stannous‐2‐ethylhexanoate, the mixture was heated at 180°C for another 3 hours under nitrogen. This is soluble in acetone but not in water.
(ii) Preparation of the Organic phase: Dissolve 150 mg of the PEG‐PLA copolymer in 8 ml acetone. Dissolve 50 mg hydrogenated soybean phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) in 4 ml ethanol with the help of Ultrasonic water bath. The two solutions are mixed to form the organic phase.
(iii) Preparation of polymerized hemoglobin: The procedure for polymerizing hemoglobin is described in details elsewhere (Chang, [Citation1997]), with the following modifications: a cross‐linker/hemoglobin optimally at a molar ratio of 17:1; 5 hours of crosslinking; and glutaraldehyde concentration of 0.5 M. The polyhemoglobin so formed is then used for PEG‐PLA nanoencapsulation. Before nanoencapsulation 0.04 ml of Tween 20 is added to 25 ml of polymerized hemoglobin at a concentration of 15 g/dl.
(iv) Nanoencapsulation: Slowly inject (8 ml/min.) (The injection head is made with a 0.2 ml pipette tips) the organic phase into the aqueous phase under magnetic stirring [with Therm‐O‐Swirl Stirrer (Precision Scientific Co., Chicago) setting to 6), at 4°C. The nanocapsules are formed immediately, but, the suspension is kept stirring for 15 min. The suspension prepared is 37 ml. The organic solvent is partly removed from the above suspension prepared by rotary evaporator under vacuum at 20°C for about 10 minutes. The suspension obtained is 33 ml (i.e. removed 4 ml organic solvent). The remained suspension is mixed with 15 ml of 0.9% NaCl.
(v) Treatment of nanocapsules with crosslinker: When applicable glutaraldehyde (0.5 M) at a molar ratio of glutaraldehyde/hemoglobin of 17:1 is added to the PEG‐PLA hemoglobin nanocapsule suspension with magnetic stirring at 4°C. After 24 hours, the crosslinking is stopped by adding 2 M of lysine at a molar ratio of lysine/hemoglobin = 100:1.
(vi) Then the organic solvent and any free hemoglobin are removed by ultrafiltration (by Amicon ZM 500,000 membrane, MW cut off 500,000) .The suspension is repeatedly washed by 0.9% NaCl by ultrafiltration. The operation is carried out at 4°C, with nitrogen to prevent methemoglobin formation.
Results and Discussions
HB Nanocapsules from a Blood Transfusion Point of View
(1) In the earlier studies (Chang and Yu, Citation[2001]; Citation[[Yu and Chang, submitted]), we were looking at the slope of the disappearing curve of the hemoglobin concentration and extrapolated to zero to calculate the circulation half‐time. This is fine for comparing and screening a large number of Hb nanocapsules prepared by different procedures. However, this type of analysis is more suitable for looking at drug delivery systems. In blood substitutes, we are more interested in the actual amount of hemoglobin that is circulating to supply oxygen. This being the case, we are more interested in the actual level of hemoglobin remaining in the circulation rather than the slope of the curve.
(2) Circulation time in rats for Hb blood substitute is known to be much shorter than in human. Thus, we need to have some baseline reference in comparing the significance of results obtained in rats to human. We do this by carrying out simultaneous studies using glutaraldehyde crosslinked PolyHb similar to those used in Phase III clinical trials in human. Knowing that with 30% blood volume infusion the circulation half time of these preparations in human is about 24 hours, we now have a direct basis for extrapolation to human. However, rats are known to have a much more avid RES in the removal of particulates like nanocapsules. Thus, the results when extrapolated to human for Hb nanocapsules may be much lower than what it would be.
Baseline Studies Using Polyhemoglobin
In order to have valid comparisons, all studies in rats use the same protocol for both polyhemoglobin solutions and Hb nanocapsules suspensions. They are adjusted to have the same hemoglobin concentration of 10 gm/dl—concentrations being used in Phase III clinical trials. The volume infused is the same for both PolyHb and Hb nanocapsules and is 30% of the total blood volume (30% top‐load).
We prepared two types of polyHb in the laboratory using our glutaraldehyde crosslinked method (Chang, [Citation1997]). One type using a crosslinker to Hb ratio of 10:1 contains higher percentage of crosslinked tetrameric Hb (). The second type using a crosslinker to Hb ratio contains a low percentage of crosslinked tetrameric Hb ().
Figure 1. Molecular weight distributions of two types of polyhemoglobin. PolyHb 10:1 prepared using a molecular glutaraldehyde to hemoglobin ratio of 10 to 1; PolyHb 17:1 using a ratio of 17 to 1.
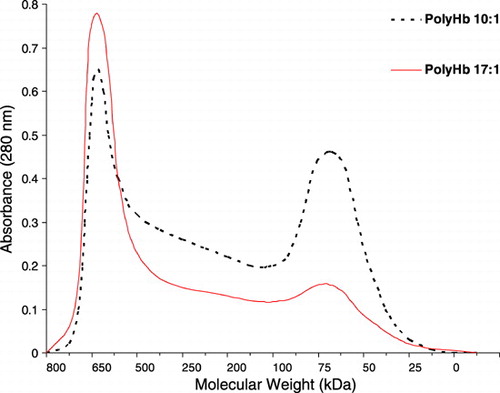
Figure 2. The maximal systemic non‐rbc hemoglobin reached after infusion and the time to reached a given non‐rbc hemoglobin level when using the 2 types of polyHb from .
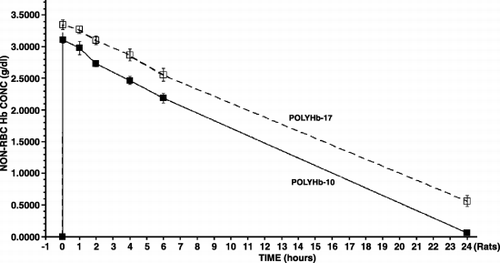
Thus shows that 30% top‐load using preparations with Hb10 gm/dl in rats resulted in: (a) Polyhemoglobin (17:1): maximal non‐rbc Hb conc. 3.35 gm/dl, falling to half its maximal concentration of 1.67 gm/dl in 14 hrs. The molecular weight distribution of PolyHb (17:1) prepared in this laboratory is similar to those with less tetramers now in Phase III clinical trials. From here on, PolyHb (17:1) is used as the basis for comparison to all other preparations including the time for the circulating non‐rbc Hb of different preparations to reach 1.67 gm/dl. In the case of PolyHb prepared with a lower ratio of glutaraldehyde (10:1), it has higher percentage of crosslinked tetrameric Hb and is more comparable in molecular weight distribution to another polyHb prepared with another crosslinker. For this, with 1/3 toploading in rats, the maximal non‐rbc Hb concentration reached is 3.10 gm/dl, falling to 1.67 gm/dl in 10.4 hrs ().
Different Types of PEG‐PLA Copolymer HB Nanocapsules
Individual or combinations of the following 4 factors are used to prepared different types of nanocapsules. These are studied in regard to the maximal systemic Hb attained and the time of maintaining a systemic non‐rbc Hb level equal to that of polyHb (17:1), 1.67 gm/dl. The four factors are as follows.
(i) Use of different degrees of polymerized Hb.
(ii) Effects of higher M.W. PLA.
(iii) Effects of concentrations of the PEG‐PLA copolymer.
(iv) Crosslinking of the newly formed PEG‐PLA Hb nanocapsules.
Effects of Molecular Weight Distribution of PolyHb Used in the PEG‐PLA Nanocapsules
30% top‐load using Hb nanocapsules containing polyHb (10:1) results in a maximal non‐rbc Hb level of only 3.05 gm/dl (S.D. = 0.03). The non‐rbc Hb falls to 1.67 gm/dl in 12.3 hours in rats(). Calculations based on body weight, blood volume, plasma volume and dilution factors shows that the maximal non‐rbc hemoglobin concentration for Hb nanocapsules should have been more than 3.6 gm/dl rather than only 3.05 gm/dl as for these Hb nanocapsules. This seems to show that a significant part (about 16%) of the infused Hb nanocapsules has been removed nearly immediately on infusion. Thus the next step is to try to prevent this.
Figure 3. The type of polyHb used for nanoencapsulation and the maximal systemic non‐rbc hemoglobin reached after infusion and the time to reached a given non‐rbc hemoglobin level.
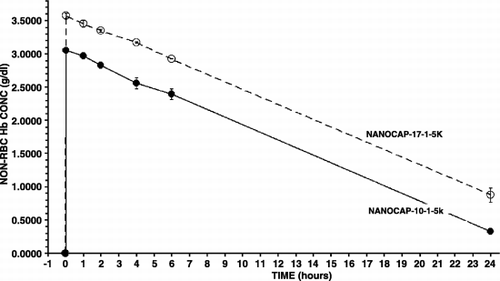
We then used a more refined method to improve the degree of polymerization to markedly reduce the amount of tetrameric hemoglobin (PolyHb 17:1) (). This is then used for nanoencapsulation. As shown in , two minutes after infusion, the maximal non‐rbc Hb is 3.58 gm/dl(S.D. = 0.04). This is significantly higher than the 3.05 gm/dl (S.D. = 0.04)for the earlier Hb nanocapsules. This also approaches the maximal possible initial non‐rbc Hb concentration. Furthermore, it took 17.1 hrs in rats for the non‐rbc Hb level to fall to 1.67 gm/dl. This very significant increase have been further improved in the following step‐wise incremental design of Hb nanocapsules until we reached a maximal concentration of 3.66 gm/dl(S.D. = 0.03) and 24.2 hrs to fall to the level of 1.67 gm/dl in rats.
Effects of Higher Concentration of PEG‐PLA Copolymer Combined with PolyHb (17:1)
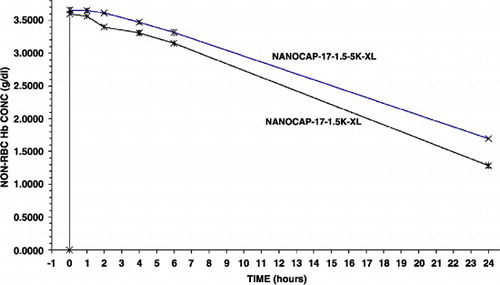
Using the same method as above with PolyHb (17:1) but with a 1.5 times higher PEG‐PLA concentration. This results in a thicker membrane with better membrane stability. This results in a further increase in circulation time (). Thus, in two minutes after infusion, the maximal non‐rbc Hb was 3.60 gm/dl(S.D. = 0.01) Furthermore, it took 20.0 hrs in rats for the non‐rbc Hb level to fall to the level of 1.67 gm/dl.
Figure 4. Variations in the molecular weights of polylactic acid (5 K or 15 K) used and the amount of polyethylene‐glyco‐polylactide copolymer used (1 or 1.5). Effects on the maximal systemic non‐rbc hemoglobin reached after infusion and the time to reached a given non‐rbc hemoglobin level.
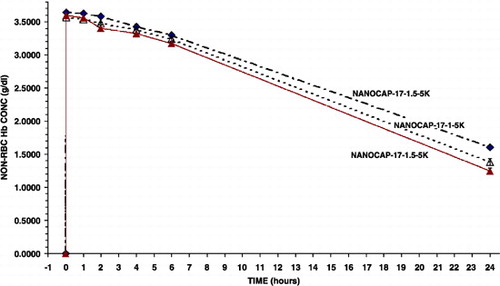
We also used a 2 times higher concentration of PEG‐PLA polymer to further improve the stability of the membrane. However, the Hb nanocapsules formed this way tend to aggregate and therefore was not tested in animal studies.
Effects of Higher Molecular Weight PLA for the PEG‐PLA Copolymer
We looked at the use of a higher molecular weight PLA to increase the stability of the Hb nanocapsule membrane. For this we replace the 5 K PLA in (1) above with a 15K PLA to form the PEG‐PLA copolymer. This also very significantly increases the circulation time of the preparation as compared to that prepared using the method in (1) () Thus in two minutes after infusion, the maximal non‐rbc Hb was: 3.57 gm/dl (S.D. = 0.05) . The slope of the disappearance was also much slower, but what is more important is that it took 21.2 hrs in rats for the non‐rbc Hb level to fall to 1.67 gm/dl.
Higher PEG‐PLA Concentration Combined with Higher Molecular Weight PLA
We next looked at combining the use of a higher molecular weight PLA (15 K) as in (3) above with a 1.5 times higher concentration of the polymer as in (2) above. This is therefore a combination of the following 3 factors:
(a) Using polyhemoglobin with low percentage of crosslinked tetrameric hemoglobin.
(b) Using a 1.5 × concentration of the PLA‐co‐PEG copolymer.
(c) Using a higher molecular weight PLA (15 K).
This resulted in a further significant improvement. In two minutes after infusion, the maximal non‐rbc Hb was: 3.6458 gm/dl(S.D. = 0.02). The slope of the disappearance was also much slower, but what is more important is that it took 23.3 hrs in rats for the non‐rbc Hb level to fall to the level of 1.67 gm/dl ()
Effect of Crosslinking the Newly Formed Hb Nanocapsules
The method described in (1) above using polyHb (17:1) was modified by adding glutaraldehyde to the Hb nanocapsules suspension after they are formed. This approach has been used earlier to stabilize both the larger Hb microcapsules and the protein inside (Chang, [Citation1971]). The polymerization was stopped by adding 2M of lysine (at molar ratio of lysine/hemoglobin = 100:1) after 24 hours. This approach also increased the circulation time to the same degree as when using 1.5 concentration of the polymer in (2) above. Thus, in two minutes after infusion, the maximal non‐rbc Hb was higher: 3.60 gm/dl (S.D. = 0.01). The slope of the disappearance was also much slower, but what is more important is that it took 20.3 hrs in rats for the non‐rbc Hb level to fall to 1.67 gm/dl ().
Effects of Combination of All 4 Factors to Prepare Hb Nanocapsules
Finally, we combined all the above 4 factors as follows:
(a) Using the polyhemoglobin (17:1) with low percentage of single crosslinked tetrameric hemoglobin.
(b) Using a 1.5 × concentration of the PLA‐co‐PEG copolymer.
(c) Using a higher molecular weight PLA (15K).
(d) Crosslinking of the newly formed Hb nanocapsules with glutaraldehyde.
In two minutes after infusion, the maximal non‐rbc Hb was higher at 3.6583 gm/dl (S.D. = 0.03) (). The slope of the disappearance was also slower, but what is more important is that it took 24.2 hrs in rats for the non‐rbc Hb level to fall to 1.67 gm/dl.
Analysis of Results
All the results obtained are summarized into one figure (). These show clearly that 2 minutes after infusion the maximal Hb concentrations attained with the better Hb nanocapsules are the maximal levels possible. After a 30% blood volume toploading using Hb concentration of 10 gm/dl, the best polyHb can only attain a maximal Hb concentration of 3.35 gm/dl. The best PEG‐PLA Hb nanocapsules, on the other hand, can reach a maximal Hb concentration of 3.66 gm/dl (). This cannot be explained by polyHb having more colloid osmotic pressure than Hb inside nanocapsules resulting in some hemodilution. After all, in those PEG‐PLA Hb nanocapsules that are removed more rapidly than PolyHb‐17, e.g. nanocap‐10‐1‐5 K, the maximal Hb reached for nanocap‐10‐1‐5 K is lower than that of PolyHb‐17 ( and ). The more likely explanation is that for the best PEG‐PLA Hb nanocapsules, they all remain in the circulation when the first samples are taken at 2 minutes after infusion. For nanocap‐10‐1‐5 K and PolyHb, a very small fraction must have been removed within 2 minutes after infusion.
Figure 6. Analysis of results from above. PolyHb‐17 is used as the standard for extrapolation of the results obtained for the different types of Hb nanocapsules. The time for PolyHb‐17 to reach a non‐rbc hemoglobin level of 1.67 gm/dl is 14 hours in rats equivalent to 24 hours in human. The time to reach this non‐RBC hemoglobin concentration of 1.67 gm/dl is used to analyze the time for Hb naoocapsules to reach this level. This is then used to calculate the equivalent time for human.
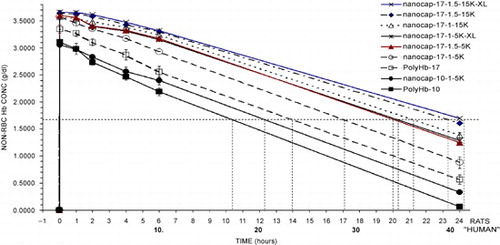
Table 1. Summary from the Analysis of Results
After a 30% blood volume toploading using Hb concentration of 10 gm/dl, the best polyHb can maintain a systemic hemoglobin level reaching 1.67 gm/dl after 14 hours in rats (equivalent to about 24 hours in human). In the case of the best Hb nanocapsules a similar toploading can maintain a much longer systemic Hb level reaching 1.67 gm/dl after 24.2 hours in rats (). If one uses the rat results in this study for polyHb of 14 hours and its clinical equivalent of about 24 hours, we might calculate and extrapolate this as follows. For the best PEG‐PLA Hb nanocapsules with the ability to maintain a systemic Hb level reaching 1.67 gm/dl after 24.2 hours, it is likely equivalent to about 41.5 hours in human. ( and ).
Thus, the equivalent functional circulation time in human after 30% toploading using the best PEG‐PLA Hb nanocapsules could be 41.5 hours in human when compared to 24 hours for glutaraldehyde crosslinked polyHb. This is a very significant and important increase that would allow longer function after infusion and thus decrease the need for donor blood and further increase the avoidance of donor blood. The Hb nanocapsules are likely to have even higher circulation time in human compared to PolyHb. This is because the reticulo‐endothelial systems in rats are much more efficient in removing particulates like nanocapsules as compared to PolyHb solution. A further advantage of PEG‐PLA nanoencapsulation of polyHb is that even if PolyHb leaks slowly out of nanocapsules after infusion it would continue to act and, unlike tetrameric Hb, would not cause adverse effects. Indeed, PEG‐PLA nanocapsules, could be an useful carrier for other modified Hb including recombinant Hb, PEG conjugated Hb to prolong further their circulation time, inclusion of enzyme systems and avoid direct external exposure.
Prevention of Methemoglobin Formation
By increasing the circulation time, we have significantly increased the in‐vivo functioning of the hemoglobin in the nanocapsules. However, with increase in circulation in the body at 37°C, there would be a steady increase in methemoglobin. Oxidation of hemoglobin to methemoglobin inside red blood cell is prevented by the enzyme systems of the red blood cells (rbc).
Hb Nanocapsules Containing metHb Reductase System
Hb nanocapsules are prepared to contain all the enzymes of the red blood cells (). This is done by extracting from red blood cells all the enzymes and hemoglobin. We prepared Hb nanocapsules with higher methemoglobin level of 7%. We then incubated these at 37°C and follow the changes in the % of methemoglobin (metHb) under the following 2 conditions:
(i) When suspended in Ringer lactate containing 100 mg/dl glucose, metHb in the Hb nanocapsules increased by a total of 2.5% in 6 hours.
(ii) When suspended in Ringer lactate containing 100 mg/dl glucose and 0.02 mM NADH, As glucose and NADH entered the nanocapsules to start the multienzyme reaction, instead of an increase, metHb was decreased by 1.5% in 5 hours. This result is very exiting because it shows that we only need to encapsulate fresh red blood cell contents with the normal amount of methemoglobin reductase system. This way, 100 mg glucose (available as blood glucose) and 0.02 mM NADH in the suspending medium not only prevent metHb formation, but can convert preformed metHb back to Hb. By further optimization of the NADH concentration, this can be increased further.
Figure 7. Nanodimension artificial red blood cells containing red blood cell enzymes including methemoglobin reductase system, carbonic anhydrase, catalase and superoxide dismutase. Methemoglobin formation can be reduced by either the more complicated methemoglobin reductase system or by plasma reducing agents diffusing into the nanocapsules.
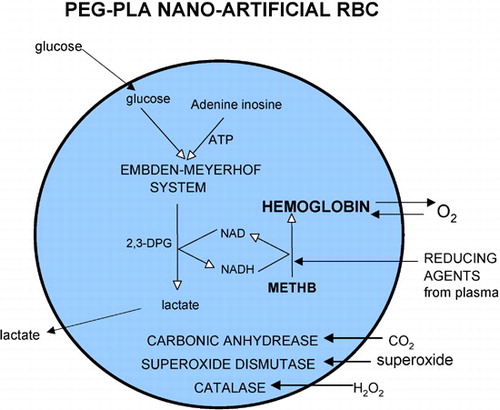
Unlike NADH, the larger cofactor NADPH is not permeable across the nanocapsules. Since NADPH is not permeable, it can be enclosed inside the nanocapsules. This would avoid the need to supply external cofactor and allow the reaction to take place like in the RBC.
Hb Nanocapsules Permeable to Reducing Factors from Plasma
There are reducing agents in the plasma that prevent methemoglobin formation. Examples include ascorbic acid and glutathione. Unlike Hb liposomes, Hb nanocapsules are permeable to these and other plasma reducing molecules. We suspended Hb nanocapsules in solutions of ascorbic acid, glutathione, or methylene blue. The Hb nanocapsule membrane is permeable to all these factors. The Hb in the nanocapsules will therefore be exposed to reducing factors in the circulating plasma that prevent the formation of metHb. This is an additional mean of preventing the formation of methemoglobin that can be used to form a simpler system.
Other Red Blood Cell Enzymes
Other red blood cell enzymes present in the red blood cell extract, like carbonic anhydrase, catalase and superoxide dismutase are also included in the PEG‐PLA Hb nanocapsules (). In addition, higher concentrations of rbc enzymes like catalase and superoxide dismutase can be included. Other non‐rbc enzymes can also be included.
Acknowledgments
TMSC is responsible for the stepwise design of nanocapsules preparation, planning of experiments, interpretation and analysis of results, writing the manuscripts and the discussions for this paper. DP is responsible for carrying out the preparation and animal studies. This research is supported by the following peer review competitive awards to TMSC: Mainly from the Joint Research Fund from Bayer/Canadian Institutes of Health Research/Canadian Blood Agency/HemaQuebec including the research assistant position for DP; but also from Canadian Institutes of Health Research; “Virage” Centre of Excellence in High Technology award of the Quebec Ministry of Higher Education, Science and Technology; MSSS‐FRSQ Research Group (equipe) on “Blood Substitutes in Transfusion Medicine,” Quebec Ministry of Health's new “Hemovigillance and Transfusion Medicine Program.”
References
- Alayash Al. Hemoglobin‐based blood substitutes: oxygen carriers, pressor agents, or oxidants?. Nature Biotechnol. 1999; 17(6)545–549
- Chang T. M.S. Semipermeable microcapsules. Science 1964; 146(3643)524–525
- Chang T. M.S. Stabilization of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross‐linking with glutaraldehyde. Biochem. Biophys. Res. Commun. 1971; 44(6)1531–1536
- Chang T. M.S. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J. Bioeng. 1976; 1: 25–32
- Chang T. M.S. Monograph on “Blood Substitutes: Principles, Methods, Products and Clinical Trials”. Basel Karger, Texas Landes 1997; Vol. 1, (free viewing on www.artcell.mcgill.ca)
- Chang T. M.S. Oxygen carriers. Curr. Opin. Invest. Drugs 2002; 3(8)1187–1190, (free viewing on www.artcell.mcgill.ca)
- Chang T. M.S., Yu W. P.. Biodegradable Polymer Membrane Containing Hemoglobin for Blood Substitutes. U.S.A. Patent 5670173, September 23, 1997
- Chang T. M.S., Yu W. P.. Biodegradable Polymeric Nanocapsules and Uses Thereof. U.S. Provisional Patent Application. No 60/316,001, August 31, 2001
- Chang T. M.S., Powanda D., Yu W. P.. Biodegradable Polymeric Nanocapsules and Uses Thereof. International Patent Application No. PCT/CA02/01331, August 2002
- D'Agnillo F., Chang T. M.S. Polyhemoglobin‐superoxide dismutase‐catalase as a blood substitute with antioxidant properties. Nature Biotechnol. 1998; 16(7)667–671
- Gould S. A., Moore E. E., Hoyt D. B., Burch J. M., Haenel J. B., Garcia J., DeWoskin R., Moss G. S. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J. Am. Coll. Surg. 1998; 187: 113–120
- Sprung J., Kindscher J. D., Wahr J. A., Levy J. H., Monk T. G., Moritz M. W., O'Hara P. J. The use of bovine hemoglobin glutamer‐250 (Hemopure) in surgical patients: results of a multicenter, randomized, single‐blinded trial. Anesth. Analg. 2002; 94(4)799–808
- Squires J. E. Artificial blood. Science 2002; 295(5557)1002–1005
- Yu W. P., Chang T. M.S. Nano‐dimension red blood cell substitutes based on a novel ultrathin polyethylene‐glycol‐polylactide copolymer membrane nanocapsules containing hemoglobin and enzymes, (submitted)