Abstract
Formulation of membrane properties is important prior the successful implantation of encapsulated cells producing therapeutically relevant compounds. The purpose of our study was to specify the methods allowing preliminary evaluation of hollow fibers (HF) chosen for immunoisolation. We have selected as estimates (1) diffusive permeability for small and large solutes, and HF cut off (in vitro), (2) histological evaluation of tissue overgrowth after sc. implantation into mice. It was found that diffusive coefficients were linearly dependent on the particle diameter except that of albumin (2–3 times higher than theoretically estimated). This discrepancy imply that for certain particles the interaction with membrane material may be significant. The histological evaluation showed that siliconized HF implanted for 105 days were accepted (there was thin fibrotic layer on the external surface of the HF, no surrounding haemopoietic cells were found). It is concluded that proposed methods for preliminary evaluation of hollow fibers chosen for immunoisolation seems to be reliable and suitable for testing diffusive permeability of each relevant cell product.
Introduction
The rapid development of biotechnology allows to use encapsulated in membranes cells as a system for production of factors necessary for host functioning. The HF for cell immunoisolation should separate encapsulated cells from host immune system cells, and exhibit sufficient permeability for nutrients necessary for cell survival and/or proliferation.
The polypropylene HFs for filtration use were chosen for the experiments, because of their cut –off and good mechanical resistance. The purpose of our study was to examine the stimulation of tissue overgrowth around the HFs, during implantation and to evaluate the HF's permeability properties, using the methods which would allow to assess the HF's usefulness for immunoisolation.
Evaluation of Diffusive Transport In Vitro
Materials
Hollow fibers of polypropylene K600 PP, Accurel (Akzo‐Nobel, Wuppertal, Germany), inner diameter 0.6mm, wall thickness 0.2mm, pore diameter 0.5µm; Reagents: creatinine (Sigma, Germany, 99% purity), bovine albumin (KWSS, Cracow, Poland, 95% purity), bovine IgG (WWSS, Warsaw, Poland, 98% purity), myosin (from rabbit muscle, Sigma, USA), human albumin (crystallized; Sigma, USA), radiolabeled (125I‐hAlb); SA 30 TBq/mmol) was prepared as a marker allowing its direct quantification (microchloramine T method); physiological saline.
Methods
Preparation of the siliconized HF: The solution of silicone oil was introduced into a membrane structure by its exposure to the solvent in pressure compartment (‐0.1MPa÷0.1MPa) in temperature 20°C for 5–60 minutes. The solvent may be aliphatic or aromatic hydrocarbons, alcohols, aldehydes, ketones, amines. It was observed by electron microscopy that exposure time and pressure had no significant effect on silanization of the membrane surface. Initially different viscosities were tested: 2200–3700 cSt and concentrations 0.1–12.2%. The best results were obtained for viscosities 2700–3000 cSt, concentration 1–2%, temperature 20°C, time of exposure 15 minutes ( and ). The HF pore size obtained after silanization, evaluated using “bubble point” method (Reichelt, [Citation1991]) was 0.47 ± 0.03µm (n = 10).
Figure 1. The surface of the hollow fiber of polypropylene K600®PP, Accurel, Akzo original (× 1000).
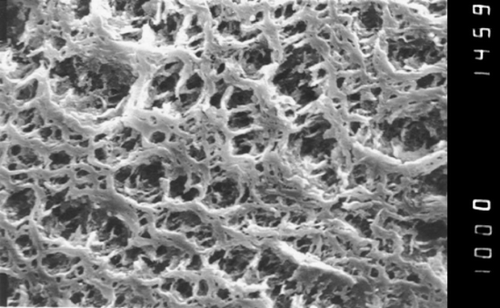
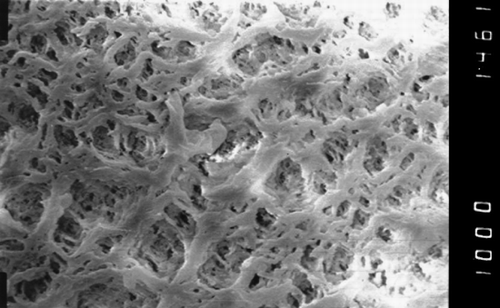
Figure 2. The surface of the hollow fiber of polypropylene K600®PP, Accurel, Akzo modified by siliconization (× 1000).
Evaluation of diffusive permeability. To evaluate the diffusive permeability of hollow fibers the thermodynamic description of diffusive mass transport (Fick's law) and a 2‐compartment model were applied as described previously (Granicka et al., [Citation1996]). Shortly, the HF were stored for 30 minutes in 70% ethanol and then washed for15 minutes in distilled water followed by washing in physiological saline. The HF were filled with tested solute solution in saline, sealed on the both ends and immersed in a beaker with 5 ml continuously mixed physiological saline. The samples were taken from the beaker at various time periods to evaluate the solute concentration.
The polypropylene K600 HF permeability was measured for labeled 125I human albumin (125I‐hAlb). The different solute concentrations were examined, from the low to physiological ones, to check whether the osmotic pressure caused by high concentrations does not increase the diffusive permeability (P) value. The HF permeability was also evaluated, for creatinine, bovine albumin, bovine IgG and myosin.
The HF's effective area (A) and volume were about 0.002 m2 and 0.1 ml respectively. The tested initial solute concentrations in saline (0.9% NaCl in water) were for: creatinine 100 mg/dl, bovine albumin 5g/dl, bovine IgG 4g/dl, myosine 40 mg/dl, and for hAlb 0.1–2.0 g/dl (with addition of about 2.4 × 106 cpm of 125I‐hAlb per HF) Concentration of : creatinine was read directly at 235 nm; bovine albumin IgG and myosin were read directly at 280 nm in the spectrophotometer Shimadzu UV‐160 (Shimadzu Corporation, Japan). Concentration of 125I‐hAlb was evaluated by measurement of samples activity in gamma ray counter (Gamma 5, Becton‐Dickinson, USA).
The permeability evaluation using single HF system allows to obtain relevant data for cell‐encapsulated systems.
To compare the diffusive permeability values obtained for single HF, the diffusive permeability derived from clearance value was evaluated using dialyser manufactured in our laboratory with the examined HFs.
The parameters of dialyser were as follows: hollow fibers of polypropylene K600 PP Accurel: inner diameter 0.6 mm, wall thickness 0.2 mm, length 140 mm, effective area 0.056 m2.
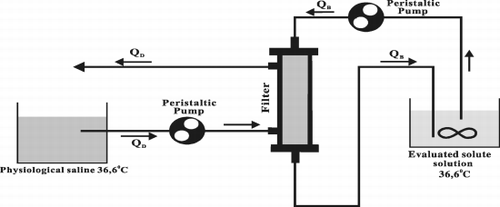
The dialyser circuit is presented in . The circuit consists of two loops: closed “blood” loop, where the solution of tested solutes simulated blood was pumped and dialysate (physiological saline) circuit. All the experiments were performed in 37 ± 1°C, the rate of “blood” flow QB to dialysate flow QD was constant and was equal to 0.4. The set value was for: “blood” QB = 50ml/min, dialysate QD = 125ml/min. All the experiments were performed in condition of pure diffusion (ultrafiltration, and transmembrane pressure (PTM) were equal to zero). Those conditions responded to transmembrane pressure defined as: PTM = (pB1 + pB0)/2‐(pD1–pD0)/2 where: pB1,pB0 [mmHg]‐inlet and outlet “blood” (here: evaluated solute solution) pressure; pD1, pD0 [mmHg]‐inlet and outlet dialysate pressure.
The PTM = 0 was achieved by regulation of hydrostatic pressure. The period of experiment in established flow rate of solutions was about 60 minutes. The samples from the inlet and from the outlet “blood” were taken every 15 minutes to evaluate the concentrations CBin, CBout . The concentration of examined solute solutions was evaluated using colorimetric tests in spectrophotometer Shimadzu UV 160. The value of diffusive clearance [ml/min] was determined using following equation:Using the equation for clearance (Weryński and Waniewski, [Citation1995])
where:
QB‐“blood” flow velocity [ml/min]; QD‐flow velocity of dialysate [ml/min]; A‐ effective dialyser surface area [m2]; P‐ diffusive permeability[ml·min− 1 m− 2]the diffusive permeability was evaluated:
Evaluation of Tissue Overgrowth
Materials
Hollow fibers of polypropylene K600 PP, Accurel: original or siliconized.
The HF were sterilized as mentioned above.
Animals. Mouse CDF1 (BALB/c × DBA/2)F1, body weight about 20g, on standard diet and water ad libitum were used. The protocol for animal experiments was examined and approved by the local ethical committee.
Methods
HF of polypropylene K600 original or siliconized were implanted subcutaneously into anesthetized mice and kept for 52 or 105 days. After explantation the macroscopic and microscopic evaluation of implants was performed. The HF's surface was examined histologically with hematoxyline and eosin to observe the tissue overgrowth.
Results and Discussion
Evaluation of Membrane Diffusive Permeability
The diffusive permeability for creatinine, bovine albumin, human albumin 125I labeled, IgG and myosine was evaluated. The obtained values for creatinine, IgG, myosine, albumin bovine and 125I‐hAlb are presented in
Table 1. Diffusive Permeability Values for Polypropylene K600 PP, Accurel Original or Silikonized HF for Creatinine, Bovine Albumin, Human Iodinated Albumin, IgG and Myosin Measured for Single HF and for HF in Module. The Values of the Rate of HF Diffusion Coefficient to Water Coefficient for Examined Solutes (Dmem/DH2O)
To compare the diffusive permeability P measurement for creatinine and albumin obtained for single HF, the module of K600 original HF was prepared and examined as a dialyser. The applied flow velocities were: blood 50ml/min, dialysate (0.9%NaCl) 125ml/min; effective HF surface area was 0.056 m2. The creatinine clearance was 3.28 ± 0.32 [ml/min]. The diffusive permeability was calculated using Eqn 2. The permeability [cm/s] (mean ± SD) was 1072 ± 98, (n = 4). The permeability for single K600 HF was 1034 ± 55 (n = 3). The diffusive permeability values obtained with both methods: for single HF and for dialyser, were very close, differed only by 1%. The permeability value for albumin was 320 ± 17 (n = 3) and differed significantly from the permeability values obtained for single HF. Application of dialyzer diminishes the impact of boundary layers resistances for diffusion, for large solutes, increasing the permeability values.
In single HF evaluation, the values obtained for siliconized HF were comparable for 125I‐hAlb and for bovine albumin. The values of the ratio of diffusive coefficient for albumin in water and in the original membrane significantly differed from expected ones as well as in siliconized membranes.
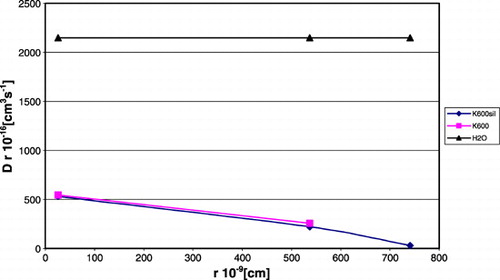
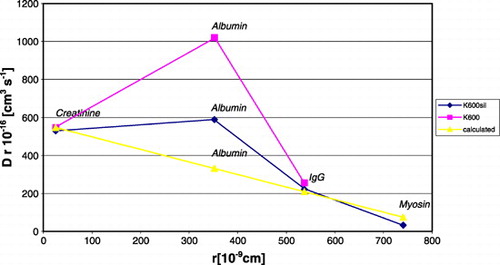
The presents the product of diffusive coefficient and particle radius as a function of particle's radius during transport through the water or through the evaluated membrane, for creatinine, IgG, myosine. The product of experimental, diffusive coefficient and particle's radius values for selected particles: in membranes presented rectilinear dependence on the particle radius; in water was constant.
Figure 4. The product of diffusion coefficient and particle radius in the function of particle radius in water and in permiselective membrane for creatinine, IgG, myosine.
The experimental values obtained for albumin disagreed with rectilinear dependence obtained for creatinine, IgG, myosine. The presents the product of diffusion coefficient and particle radius as a function of particle's radius for albumin, calculated and experimental. As can be inferred from , the diffusion coefficient for albumin (r = 352.1 × 10− 9 cm) in K600 membrane should be equal to 0.9 × 10− 7[cm2s− 1]. The obtained diffusion coefficient value for K600 is almost 3.5 times higher than theoretically estimated, for K600 siliconized is two times higher.
Figure 5. Relationship between particle radius and the product of particle radius and diffusion coefficient, calculated and experimental.
The adsorption of albumin may diminish the ratio of diffusive coefficient for albumin in water and in the applied membranes meanly up to 20% (data not presented in the table).
Evaluation of Tissue Overgrowth
The overgrowth with connective tissue cells in vivo was observed on the surface of implanted polypropylene K600 PP original and surface modified HF.
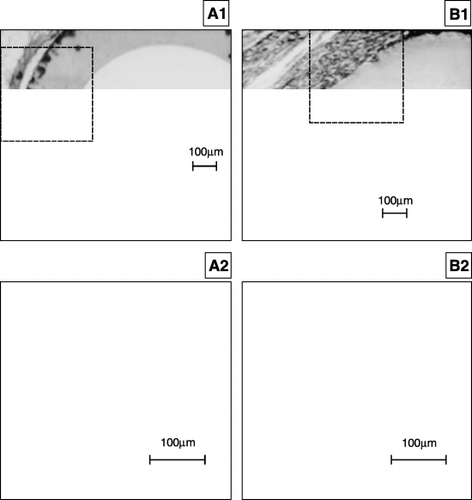
The explanted HF were examined histologically after 52 or 105 days of implantation. In case of 52 days implantation K600 original and surface modified K600 implants were accepted by the host. There was thin fibrotic layer on the external surface of the HF, no haemopoietic cells were found. After 105 days of implantation the external surface of K600 siliconized was surrounded by the thin layer of fibroblasts (thickness about 30µm), there was calcium salt intrusion in the membrane wall. There was a slit between the fibroblasts layer and the external HF surface. The implant appeared to be accepted (). In case of K600 original, there was foreign body reaction on the surface of the HF. The presence of macrophages, fibroblasts and lymphocytes was observed ().
Figure 6. A1, A2. The view of the K600 siliconized HF surface after 105 days implantation into mouse. The external surface of HF was surrounded with the thin layer of fibroblasts (thickness about 30µm), there was calcium salt intrusion in the membrane wall. There was a slit between the fibroblasts layer and the external HF surface. The implant appeared to be accepted (× 70). B1, B2. The view of the K600 original HF surface after 105 days implantation into mouse. There was foreign body reaction on the surface of the HF. The presence of macrophages, fibroblasts and lymphocytes was observed (× 70).
The diffusive permeability evaluation for single HF is a simple, relatively cheap method of membrane transport assessment. Presuming that the HF cut off is equal to the solute molecular weight when the ratio of diffusive coefficient in HF to diffusive coefficient in water (DM/DH2O) for that solute is equal or lower than 5%, the diffusive cut off of K600 siliconized is about 200 kD.
The obtained permeability values for examined HF were compared with permeabilities of membranes used by other authors for immunoisolation. Dionne et al ([Citation1996]) evaluated poly(acrylonitrile‐co‐vinyl chloride) HF diffusive permeability for different solutes with the nominal molecular weight cut‐off of 80 kD. The ratio of diffusive coefficient in that HF to diffusive coefficient in water (Dm/DH2O) for IgG was about 0.04%, for bovine albumin 0.06%. Morikawa et al. ([Citation1997]) evaluated diffusive permeability of poly(2‐hydroxyethyl methacrylate) microcapsules. The diffusive coefficient for bovine albumin was about 0.01 × 10− 7 cm2s− 1. The calculated ratio Dm/DH2O was 0.1–0.2%. In our experiments, the ratio DM/DH2O [%] obtained for albumin and IgG using polypropylene original, and siliconized K600 were: 47.5, 11.9 and 31.5, 10.3 respectively.
The polypropylene HF evaluated experimentally were permeable for large solutes as found in this and our previous studies (Granicka et al., [Citation1996]; Granicka et al., [Citation2000]), however in opinion of many authors (Aebischer et al., [Citation1994]; Altman et al., [Citation1984]; Lanza et al., [Citation1995]; Maki et al., [Citation1991]; Monaco and Maki, [Citation1996]), the HF should be impermeable to immunoglobulins and complement components in order to avoid the immune attack. Nevertheless Lanza et al. ([Citation1995]) used successfully alginate, permeable for particles of MW over 600 kD for encapsulation of pig Langerhans islets. Immunohistological staining of long‐term islet grafts in mice (over 10 wk) revealed viable cells, and the external surfaces of the microreactors were free of tissue overgrowth. In short time experiments with implantation of different cell types encapsulated in polypropylene siliconized HFs (cut off 200 kDa), the cells were viable (Granicka et al., [Citation1996]; Granicka et al., [Citation1999]).
To recognize the antigen presented in MHC class I complex by T lymphocytes, the direct contact is indispensable. The membrane used in the experiments has cut‐off about 200 kDa and is the barrier to the cells allowing to avoid the direct contact between cytotoxic T‐lymphocytes and transplanted cells. In humoral response, the complement activation is necessary to initiate the destruction of the foreign cell. However the passage of the first element of the complement C1q protein (MW 410 kDa) through the membrane probably can not be effective. The alternative pathway post C3 necessitate properdin (P) (MW 224kD) for stabilization of convertase C3/C5. In absence of P, convertase C3/C5 is not protected from factors H and I, what makes impossible the membrane attack complex (MAC) forming (which enable the cell destroying).
The discrepancy between experimental and theoretically estimated values for albumin may be explained by that, there is probability of lower tendency to surface deposition of hydrophilic albumin (dehydrated in water solution) on hydrophobic polypropylene membrane surface than on hydrophilic membranes (like polyamides, regenerated cellulose), what can effect in increasing sieving for albumin (Clark et al., [Citation1995]; Conti et al., [Citation2002]).
Electrostatic interactions with the membrane material cannot also be excluded. Such an effect was recently described for albumin by Menon and Zydey in terms of an effective protein radius, which accounts for the electrostatic exclusion of the charged protein from the membrane pores (Menon and Zydney, [Citation1999]). Although the results for all other solutes tested are in good agreement with theoretical estimates, it is postulated that the experimental analysis is necessary when evaluating specific membrane material and the particle in question.
Conclusions
The diffusive coefficients were used as the criteria of membrane evaluation for two systems (single HF and HF in module) with macro – and microscopic evaluation of preparations. K600 siliconized HF seems to be most suitable for cells encapsulation. The divergence of values obtained for albumin indicates need for testing the electrostatic interactions in case of this particle.
References
- Aebischer P., Buchser B., Joseph J. M., Favre J., Tribolet N., Lysaght M., Rudnick S., Goddard M. Transplantation in humans of encapsulated xenogenic cells without immunosuppression. Transplantation 1994; 58(11)1275–1277
- Altman J. J., Houlbert D., Chollier A., Ledue A., McMilian P., Gailetti M. Encapsulated human islet transplants in diabetic rats. Trans. Am. Soc. Artif. Intern. Organs 1984; 30: 382–386
- Clark W. R., Macias W. L., Molitoris B. A., Wang N. H. Plasma protein adsorption to highly permeable hemodialysis membranes. Kidney Int. 1995; 48(2)481–488
- Conti M., Donati G., Cianciolo G., Stefoni S., Samori B. Force spectroscopy study of the adhesion of plasma proteins the surface of a dialysis membrane: role of the nanoscale surface hydrophobicity and topography. J. Biomed. Mater. Res. 2002; 61(3)370–379
- Dionne K. E., Cain B. M., Li R. H., Bell W. J., Doherty E. J., Rein D. H., Lysaght M. J., Gentile F. T. Transport characterization of membranes for immunoisolation. J. Biomater. Sci. 1996; 17(3)257–266
- Granicka L. H., Kawiak J., Głowacka E., Weryński A. Encapsulation of OKT3 cells in hollow fibers. ASAIO J. 1996; 42(5)M863–M866
- Granicka L. H., Migaj M., Zawitkowska T., Woźniewicz B., Tołłoczko T., Weryński A., Kawiak J. Evaluation of human parathyroid cells functioning encapsulated in polypropylene hollow fibers. Cell Transform. 1999; 8(2)165
- Granicka L. H., Migaj M., Woźniewicz B., Zawitkowska T., Tołłoczko T., Weryński A., Kawiak J. Encapsulation of parathyroid cells in hollow fibers: a preliminary report. Folia Histochem. Cytobiol. 2000; 38(3)129–131
- Lanza R., Ecker D., Kuhtreiber W., Staruk J. E., Marsch J., Chick W. L. A simple method for transplanting discordant islets into rats using alginate gel spheres. BioHybrid Technol. 1995; 59: 1485–1487
- Lanza R., Kuhtreiber W., Ecker D., Staruk J. E., Chick W. L. Xenotransplantation of porcine and bovine islets without immunosuppresion using uncoated alginate microspheres. Transplantation 1995; 59: 1377–1384
- Maki T., Ubhi C. S., Sanchez‐Farpon H., Sullivan S. J., Borland K., Muller T. E., Solomon B. A., Cick W. L., Monako A. P. The biohybrid Artificial Pancreas for treatment of diabetes in totally pancreatectomized dogs. Transplant. Proc. 1991; 23(1)754–755
- Menon M. K., Zydney A. L. Effect of ion binding on protein transport through ultrafiltration membranes. Biotechnol. Bioeng. 1999; 63(3)298–307
- Monaco A. P., Maki T. Islet transplantation using immunoexclusion methods. Transplant. Proc. 1996; 28: 2042–2045
- Morikawa N., Iwata H., Matsuda S., Miyazaki J. I., Ikada Y. Encapsulation of mammalian cells into synthetic polymer membranes using least toxic solvents. J. Biomater. Sci. 1997; 8(8)575–586
- Reichelt G. Bubble point measurements on large areas of microporous membranes. J. Membr. Sci. 1991; 60: 253–259
- Weryński A., Waniewski J. Theoretical description of mass transport in medical membrane devices. Artif. Organs 1995; 19(5)420–427