Abstract
Methoxypoly(ethylene glycol)‐b‐poly‐DL‐lactide (PELA) microcapsules containing bovine hemoglobin (bHb) were prepared by a W/O/W double emulsion‐solvent diffusion process. bHb solution was used as the internal aqueous phase, PELA/organic solvent as the oil phase, and polyvinyl alcohol (PVA) solution as the external aqueous phase. This W/O/W double emulsion was added into a large volume of water (solidification solution) to allow organic solvent to diffuse into water. The optimum preparative condition for PELA microcapsules loaded with bovine hemoglobin was investigated. It was found that homogenization rate, type of organic solvent, and volume of the solidification solution influenced the activity of bovine hemoglobin encapsulated. When the homogenization rate was lower than 9000 rpm and ethyl acetate was used as the organic solvent, there was no significant influence on the activity of hemoglobin. High homogenization rate as 12 000 rpm decreased the P50 and Hill coefficient. Increasing the volume of solidification solution had an effect of improving the activity of microencapsulated hemoglobin. The composition of the PELA had the most important influence on the success of encapsulation. Microcapsules fabricated by PELA with MPEG2k block (molecular weight of MPEG block: 2000) achieved a high entrapment efficiency of 90%, better than PLA homopolymer and PELA with MPEG5k blocks. Hemoglobin microcapsules with native loading oxygen activity (P50 = 26.0 mmHg, Hill coefficient = 2.4), mean size of about 10 µm, and high entrapment efficiency (ca. 93%) were obtained at the optimum condition.
Introduction
Hemoglobin (Hb) is an oxygen‐carrying protein composed of four polypeptide subunits. When free Hb is injected into a body, it rapidly dissociates into toxic dimers, which are easily filtered by the kidneys, leading to renal toxicity and other adverse effects in the body (Chang, [Citation1999]).
To overcome the problems, hemoglobin used as a red blood cell substitute must be chemically modified or microencapsulated to prevent its dissociation in the plasma (Chang, [Citation1998a]). Modified hemoglobins such as crosslinked Hb, polyHb, and conjugated Hb have gone through different stages of clinical trials. Each of these will most likely be best for certain specific applications (Tsuchida, [Citation1998]).
Microencapsulated Hbs can be divided into liposome encapsulated Hb (LEH) and biodegradable microcapsule entrapped Hb. The literature of LEH has increased rapidly in these years (Rudolph and Phillips, [Citation1998]), but there are also defects to be further overcome, such as the relative instability of bilayer lipid membrane in storage and after infusion, the potential side effects of a large amount of lipid on the reticuloendothelial systems (Chang, [Citation1998b]).
Compared with LEH, the publication on microencapsulation of Hb with biodegradable polymer is rather limited. Theoretically, a biodegradable polymer microcapsule containing Hb is more like a red blood cell than a liposome containing Hb (Chang, [Citation1997]). It has a better physical strength, a better permeability, and more encapsulation content than the LEH. However, preparation of the polymer microcapsules involves tedious operations and tricks.
The reports on polymer microencapsulation of Hb came mainly from two groups. Chang's group was the first in polymer encapsulation of Hb. They used a biodegradable polymer, polylactic acid (PLA), to prepare artificial cells by a double emulsion‐solvent evaporation technique in 1976 (Chang, [Citation1976]). Few years ago, the group made a great progress in preparation of nano size capsules of Hb. Through emulsion‐solvent displacement and the emulsion‐polymerization, nanocapsules in the range of 80–120 nm were obtained (Yu and Chang, [Citation1994], [Citation1996]). Cedrati et al was the other group (Cedrati et al., [Citation1994], [Citation1997]). They used the double emulsion‐solvent evaporation process to prepare PLA (PLA) microcapsules containing human hemoglobin. A high hemoglobin encapsulation efficacy as 80% was obtained. The mean size of capsules, however, was quite large, about 150 µm.
In this paper, the amphiphilic copolymer PELA was used as wall material because of its ideal physical properties, biocompactibility and biodegradability (Chang et al., Citation[2001]). The PELA contains hydrophilic methyl polyethylene glycol (MPEG) chain block into PLA polymer to stabilize the Hb encapsulated and to increase the stability of the primary emulsion. In this way, the size of the microcapsules could be decrease while maintaining a high encapsulation efficacy. A W/O/W double emulion‐solvent diffusion process was employed to encapsulate Hb. Bovine hemoglobin (bHb) solution was as an internal aqueous phase, PELA/organic solvent as an oil phase, and polyvinyl alcohol (PVA) solution as an external aqueous phase. The W/O/W emulsion was added into a large volume of water to allow organic solvent to diffuse into the water. Compared with the evaporation process of solvent, the solvent diffusion process in this study was mild and fast. The effect of the experimental conditions, including the homogenization rate, the type of organic solvent, the volume of solidification solution, and the composition of the PELA, were investigated on oxygen‐carrying ability of the microencapsulated bHb.
Materials and Methods
Materials
The homopolymer PLA with a molecular weight (Mw) of 20 000 (g/mol) was obtained from Medical Apparatus and Instruments Co. (Shandong, China). D.L‐lactic acid (85%) was supplied by Chemical Reagent Co. (Peking, China). Poly(vinyl alcohol) (PVA Mw = 22 000, ca. 88% hydrolyzed) was purchased from Acros (Belgium). Methoxypoly(ethylene glycol) (MPEG) of Mw = 2000 (g/mol) was obtained from Fluka (Switzerland). MPEG of Mw = 5000 (g/mol) was supplied by Union Carbide (USA). Stannous octoate was purchased from Sigma (Germany). Other reagents were of analytical grade.
Stroma free bovine hemoglobin (bHb) was prepared according to the method of Doczi ([Citation1976]). Briefly, the fresh bovine blood red cell was washed with 1.6% once and 0.8% NaCl solution twice. Then, hemoglobin solution was obtained by hypotonic hemolysis with distilled water (v/v) and it was made stroma‐free by 30 000 g centrifugation followed by filtration through 0.45 µm and finally the 0.22 µm membrane. The concentration of bHb solution obtained was approximate 17 wt%.
Synthesis and Characterization of Copolymers
D.L‐latide was synthesized from D,L‐lactic acid according to the method described by Kulkarni et al. ([Citation1971]). The resulting lactide was then recrystallized three times from dry ethyl acetate, and then dried with P2O5 in vacuum.
PELA copolymer was prepared by bulk ring‐opening polymerization of D,L‐lactide and MPEG using stannous octoate as a catalyst (Deng et al., [Citation1990]). Briefly, the purified lactide and MPEG were transferred to a dry and clean ampoule, and stannous octoate solution in dried benzene was added. The bottle was evacuated and displaced with dry nitrogen gas for three times, and then placed in an oil bath at 160°C for 4 hours. To purify and remove the unreacted residuals, the crude product was dissolved in methylene chloride, and then the copolymer was precipitated by adding the solution into diethyl ether under vigorous stirring. Next, the precipitated polymer was dissolved in methylene chloride again, and then the copolymer was precipitated by adding the solution into distilled water by vigorous stirring. Two hours later, the white sediment was collected by centrifugation and dried in vacuum at 50°C over night.
The MPEG contents of copolymers were measured from the integral height of hydrogen shown in 1HNMR (DXM 300 Buker, Germany). The Mw, Mn and Mw/Mn of copolymers were evaluated by gel permeation chromatography (PL‐GPC210, England) with polystyrene as the standards, tetrahydrofuran as a elution solvent. The copolymers used in this study are indicated as PELAa(b), where a is the conceptual molecular weight of the PELA, b the MPEG block molecular weight. The characteristics of copolymers obtained are listed in .
Table 1. Charateristics of PELA
Preparation of PELA Microcapsules Containing Bovine Hemoglobin
bHb loaded microcapsule was prepared by a modified double emulsion (W1/O/W2) technique (Bouillot et al., [Citation1999]). At the first, 0.5 ml of bHb solution (ca. 17 wt%) was emulsified in 2 ml of ethyl acetate containing 30 mg of polymer by a homogenizer operating at 9000 rpm for 15 s to form the W1/O primary emulsion. This primary emulsion was immediately poured into 20 ml of an aqueous phase (W2) containing 2% (w/v) PVA and 0.9% (w/v) NaCl, and homogenized for 20 s at 5000 rpm to produce a W1/O/W2 emulsion. The double emulsion was then diluted into a solidification solution, i.e. 200 ml or 400 ml of 0.9% NaCl aqueous solution, and the system was stirred continuously by magnetic stirrer for 2 min to allow ethyl acetate to diffuse into the solidification solution, leading to microcapsule hardening. The microcapsules were then isolated by centrifugation at 9000 g for 5 min, washed three times with 0.9% NaCl aqueous solution.
Effect of Homogenization Stress on bHb Activity
2 ml of an aqueous solution of bHb (ca. 17 wt%) was stirred at different rates (5000–12 000 rpm) for 30 s. Then, the loading oxygen activity of the bHb solution was determined by a Hemox Analyzer (TCS Medical Products Co., U. S. A.).
Effect of Organic Solvent on bHb Activity
0.5 ml of bHb solution and 2 ml of organic solvent were homogenized at 8000 rpm for 30 s. Then, the bHb solution was separated from the organic solvent by centrifugation, and it's activity was obtained by a Hemox Analyzer.
Measurement of bHb Activity
The oxygen dissociation curve of a bHb sample was obtained with a Hemoxanalyzer (TCS Scientific Co.) at pH 7.4, 37°C.
Measurement of bHb Encapsulation Efficiency
The amount of bHb entrapped into the microcapsules was determined by measuring the amount of bHb in the supernatant after diffusion of the organic solvent and centrifugation. The difference between the initial amount of hemoglobin and the unencapsulated amount in the supernatant allowed the determination of the entrapment efficiency (EE) of hemoglobin in the microcapsules. The concentration of bHb in the supernatant was determined by spectrophotometry (Ultrospec 2000 Pharmacia Biotech), at 540 nm by Drabkin's method.
Measurement of Morphology and Microcapsule Size
The volume average mean size of the bHb microcapsules was measured by a Coulter Multisizer (Coulter LS230, USA). The surface morphology of microcapsules were observed with a scanning Electron Microscope (SEM) (JEOL JSM‐6700F, JPN) after coating with gold film.
Results and Discussion
Influence of Homogenization Rate on bHb Activity
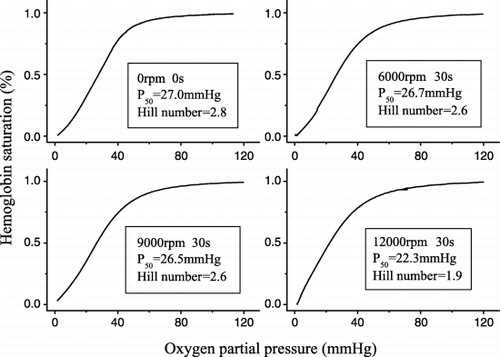
Hemoglobin is a complex protein, consisting of four subunits (2α, 2β = tetramer), and it easily dissociates into two dimers (2αβ). However, in the preparation process of bHb loaded microcapsule, emulsification is a necessary step and it is obtained by homogenization which may result in the breakdown of hemoglobin. Therefore, the influence of the homogenization rate on the activity of hemoglobin was investigated. The results are shown in . From , it was observed that the loading oxygen activity of bovine hemoglobin decreased when the homogenization rate increased. When the rate was lower than 9000 rpm, the change of the activity of hemoglobin could be neglected. When the rate increased to 12 000 rpm, the P50 and Hill coefficient of the oxygen dissociation profile of the bovine hemoglobin decreased remarkably. This implied that the conformation of hemoglobin molecules changed a lot under this homogenization stress. Therefore, to preserve the native activity of hemoglobin in the preparation process of microcapsule, the homogenization rate was maintained under 9000 rpm.
Influence of Organic Solvent on bHb Activity
Hemoglobin is a hydrophilic protein, of which surface is composed of hydrophilic groups, and the hydrophobic groups are involved in the internal of hemoglobin. When hemoglobin come into contact with organic solvent, the structure of hemoglobin may be disturbed, leading to denaturation of the hemoglobin.
In the field of double emulsion technique to prepare biodegradable microcapsule containing hydrophilic proteins, most of researchers used methylene chloride as the organic solvent (Bouillot et al., [Citation1999]; Deng et al., [Citation1999]; Pistek and Kissel, [Citation2000]; Yang et al., [Citation2001]; Zambaux et al., [Citation1999]), due to its low boiling point (39.8°C) and high solubility of biodegradable polymers. Moreover, it was reported that methylene chloride had a less denaturation effect on protein C than that of other organic solvents, especially ethyl acetate (Zambaux et al., [Citation1999]). However, the level that an organic solvent denatures a protein, not only relates to the property of the organic solvent, but also involves the intrinsic structure of the protein molecule. Thus, it is essential to select an organic solvent that has little influence on the activity of hemoglobin. In addition, the solvent used in the process must be able to dissolve the biodegradable polymer, PLA or PELA, and must be immiscible with water. The influence of the organic solvents meeting these conditions on the activity of loading oxygen of hemoglobin was evaluated, and the results are summarized in .
Table 2. The Effect of Organic Solvent on the Loading Oxygen Activity of Bovine Hemoglobin
As can be seen from , ethyl acetate, methylene chloride and trichloromethane had less denaturation effect on bHb than all the other solvents. However, the specific gravity of trichloromethane (ρ = 1.49 g/cm3) is too large to produce a stable primary emulsion, and methylene chloride (ρ = 1.33 g/cm3) has a similar problem too (Deng et al., [Citation1999]). In addition, if methylene chloride was used as the solvent, a solvent‐evaporation method must be used as the solidification technique of the bHb microcapsule, by which a longer processing time is needed. Consequently, the loading oxygen activity of the resultant bHb microcapsule decreased obviously (P50 = 14 mmHg, Hill coefficient = 1.59). The further detailed results will be given in a next paper. On the other hand, as shown in , the mixed solvent of methylene chloride and acetone (v/v), which was reported that it had a good performancement for entrapment of protein C in PLA microcapsule (Zambaux et al., [Citation1999]), was not suitable for the microencapsulation of bHb. The P50 and Hill coefficient of the bHb decreased to a low value by using the mixed solvent. For all these reasons, we chose ethyl acetate as the solvent in the preparation process of microcapsule, because it had a little influence on the activity of bHb, and it was easily removed (solubility in water, 8.7 wt% ethyl acetate in water) by a solvent diffusion method.
Influence of Volume of Solidification Solution on the Activity of Microencapsulated bHb
As mentioned above, a W/O/W double emulsion‐solvent diffusion method using ethyl acetate as an organic solvent was selected for the preparation of bHb microcapsule in this study. In the preparation process, it was found that the volume of solidification solution affected the activity of bHb remarkably. The results were shown in . When the volume of solidification solution was 200 ml, the P50 of microencapsulated bHb increased to high values, between 40 and 60 mmHg, and the Hill coefficient decreased to low values, between 1.5 and 2.0, irrespective of which copolymer wall material was used. However, when the volume of solidification solution was 400 ml, the P50 of microencapsulated bHb were about 26 mmHg and the Hill coefficient about 2.4, which were near to those of native hemoglobin.
Table 3. The Effect of the Volume of Solidification Solution on the Loading Oxygen Activity of Microencapsulated bHb
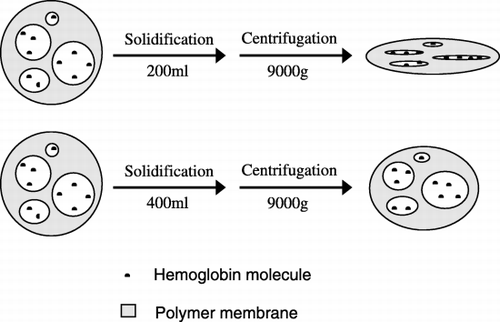
The probable reason that the volume of solidification solution influenced the activity of microencapsulated bHb is illustrated in . When the volume was lower (200 ml), although most of ethyl acetate had diffused into the outer aqueous phase, there was a little ethyl acetate remaining in the polymer membrane, and the bHb loaded microcapsules were in quasi‐solid state. Centrifuged with 9000 g at this time, the quasi‐solid microcapsules were squeezed, and the hemoglobin molecules entrapped contacted with the hydrophobic polymer membrane, resulting in the conformational change of the hemoglobin molecular and the increase of P50 of bHb entrapped. When the volume of solidification solution was higher (400 ml), the ethyl acetate remained in the microcapsule was of trace, consequently, the intensity of the polymer membrane was strong enough to resistant the press produced by centrifugation, and the contact between bHb molecules and hydrophobic polymer wall was limited. Thus, the activity of the bHb entrapped was maintained.
Influence of Polymer Type on bHb Microcapsule
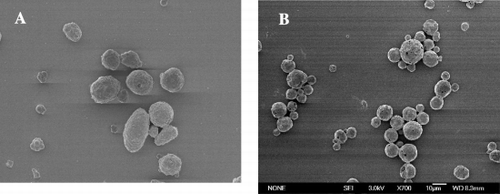
As shown in , the bHb‐loaded microcapsule (A) fabricated by PELA30k(2k) was ellipsoid, and the bHb microcapsule (B) fabricated by PELA90k(5k) was spherical with a porous surface. The morphology of microcapsules fabricated by PELA20k(2k) and PELA60k(5k) were similar with those microcapsules fabricated by PELA30k(2k) and PELA90k(5k), respectively.
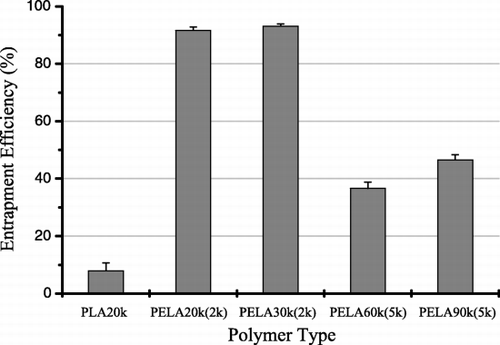
The effect of polymer wall with different molecular weight and content of MPEG on hemoglobin entrapment efficiency (EE) is shown in . The entrapment efficiency for homopolymer PLA20k was the lowest (only 7.9%) in all the five polymers, contrasted to those of PELA copolymer. The highest protein entrapped efficiency, above 90%, was obtained by using copolymers with MPEG2000 block. Other two copolymers, PELA60k(5k) and PELA90k(5k) obtained middle entrapment efficiencies, 20% and 30% respectively. These results suggested that the PELA copolymers improved protein entrapment efficiency because of their amphiphilic property. It was known that the stability of the primary emulsion was a key factor in the entrapping process (Deng et al., [Citation1999]). In primary emulsion, the MPEG block in copolymer spread into the internal bHb aqueous solution, and the PLA block stretched out into the external ethyl acetate solution. Thus, a film was formed on the interfacial between the water and oil phase by the amphiphilic copolymer, and which would limit the leakage of the internal bHb solution during the second‐step emulsification and the following solidification process, leading to a high entrapment efficiency.
As shown in , the entrapment efficiencies of bHb for the copolymers containing MPEG5k are obviously lower than those for the copolymers containing MPEG2k. These results agreed with those of Philippe Bouillot (Bouillot et al., [Citation1999]). The probable explanation was that, the stronger steric exclusion between the longer MPEG blocks decreased the stability of the film formed by the amphiphilic copolymer. Thus, the entrapment efficiency of hemoglobin decreased with the increase of MPEG block molecular weight in the copolymer. In the cases of PELA60k(5k) and PELA90k(5k), the entrapment efficiency of the latter was higher than that of the former. This observation can be explained by the effect of the viscosity of the oil phase. Under the same weight concentration, an increase in molecular weight of copolymer led to an increase in the oil phase viscosity, and then resulted in an increase in stability of the primary emulsion. Thus, a higher entrapment efficiency was obtained.
Under the same concentration, the polymer molecular weight dominated the oil phase viscosity, and the viscosity affected the disruption of the primary emulsion droplets, and thereby the resultant microcapsules size. As shown in , PELA90k(5k) has a largest molecular weight among the polymers, so that the copolymer yields a largest particle size, 11.10 µm. However, in this study, the concentration of polymer in oil phase, 15 mg/ml, was somewhat low, leading to a low oil phase viscosity. As a result, the effect of polymer molecular weight on microcapsule size was not apparent. The microcapsules with the similar particle size about 10 µm were obtained by using PELA20k(2k), PELA30k(2k) and PELA60k(5k).
Table 4. Particle Sizes and Activities of bHb Loaded Microcapsules
Finally, the loading oxygen activities of all the bHb microcapsules were near to that of native bHb, that P50 was about 26.0 mmHg and Hill coefficient 2.4.
Conclusion
The bHb‐loaded microcapsule with native activity was obtained by employing Methoxypoly(ethylene glycol)‐b‐poly‐DL‐lactide (PELA) as a polymer wall. The effects of experimental constraints such as homogenization rate, type of organic solvent, and volume of solidification solution on the loading oxygen activity of hemoglobin were investigated. The optimum condition preventing bHb from denaturation was established: homogenization rate lower than 9000 rpm, using ethyl acetate as the organic solvent, and increasing of the volume of solidification solution to 400 ml. The influence of the polymer type used as wall material on the microcapsule characteristics was studied and it was found that the PELA copolymer was more advantageous for the preparation of bHb‐loaded microcapsule than PLA homopolymer, and that the copolymers containing MPEG2000 blocks performed a successful microencapsulation of bHb.
The PELA microcapsules containing bHb with a similar mean size of 10 µm were obtained, and the loading oxygen activity of the microencapsulated bHb were near to that of native hemoglobin. All these results suggested that the process of microencapsulation did not alter the molecular structure of hemoglobin. Further research focused on reducing the particle size is being carried out in our research group.
Acknowledgments
The authors are thankful to Natural Science Foundation of China for financial support to this work (grant No. 20136020).
References
- Bouillot P., Ubrich N., Sommer F., Duc T. M., Loeffler J.‐P., Dellacherie E. Protein encapsulation in biodegradable amphiphilic microspheres. Int. J. Pharm. 1999; 181: 159–172
- Cedrati N., Maincent P., Thomas F. Preparation and characterisation of poly(lactic acid) hemoglobin microspheres. Artif. Cells Blood Substit. Immobil. Biotechnol. 1994; 22(3)867–873
- Cedrati N., Bonneaux F., Labrude P., Maincent P. Structure and stability of human hemoglobin microparticles prepared with a double emulsion technique. Artif. Cells Blood Substit. Immobil. Biotechnol. 1997; 25(5)457–462
- Chang T. M.S. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J. Bioeng. 1976; 1: 25–32
- Chang T. M.S. Recent and future developments in modified hemoglobin and microencapsulated hemoglobin as red blood cell substitutes. Artif. Cells Blood Substit. Immobil. Biotechnol. 1997; 25(1 & 2)1–24
- Chang T. M.S. Modified hemoglobin‐based blood substitutes: crosslinked, recombinant and encapsulated hemoglobin. Vox Sang. 1998a; 74(Suppl. 2)233–241
- Chang T. M.S. Nanoencapsulation of hemoglobin and red blood cell enzymes based on nanotechnology and biodegradable polymer. Blood Substitutes: Principles, Methods, Products and Clinical Trials, T. M.S. Chang. Karger, BaselSwitzerland 1998b; Vol. II: 216–231
- Chang T. M.S. Future prospects for artificial blood. Trends Biochem. Sci. 1999; 17: 61–67
- Chang T. M.S., Powanda D., Yu W. P.. Biodegradable Polymerric Nanocapsules and Uses Thereof. US Provisional Patent Application. 60/316,001, August 31,2001
- Deng X. M., Xiong C. D., Cheng L. M. Synthesis and characterization of block copolymer from lactide and poly(ethylene glycol). J. Polym. Sci., Polym. Lett. 1990; 28: 411–416
- Deng X. M., Li X. H., Yuan M. L., Xiong C. D., Huang Z. T., Jia W. X., Zhang Y. H. Optimization of preparative conditions for poly‐DL‐lactide‐polyethylene glycol microspheres with entrapped Vibrio Cholera antigens. J. Contr. Rel. 1999; 58: 123–131
- Doczi J.. Injectable Stroma‐Free Hemoglobin and Its Method of Manufacture. US Patent 3,991,181, 1976
- Kulkarni R. K., Moore E. G., Hegyeli A. F., Leonard F. Biodegradable poly(lactic acid) polymers. J. Biomed. Mater. Res. 1971; 5: 169–181
- Pistek K. F., Kissel T. Effects of salt addition on the microencapsulation of proteins using w/o/w double emulsion technique. J. Microencapsul 2000; 17(4)467–483
- Rudolph A. S., Phillips W. T. Liposome delivery of hemoglobin: molecular and cellular interactions in the vascular pool. Blood Substitutes: Principles, Methods, Products and Clinical Trials, T. M.S. Chang. Karger, BaselSwitzerland 1998; 197–205
- Tsuchida E. Perspectives of blood substitutes. Blood Substitutes—Present and Future Perspectives, E. Tsuchidaed. Elsevier Science S. A. 1998; 1–14
- Yang Y.‐Y., Wan J.‐P., Chung T.‐S., Pallathadka P. K., Ng S., Heller J. POE‐PEG‐POE triblock copolymeric microspheres containing protein I. Preparation and characterization. J. Contr. Rel. 2001; 75: 115–128
- Yu W. P., Chang T. M.S. Submicron biodegradable polymer membrane hemoglobin nanocapsules as potential blood substitutes: a preliminary report. Artif. Cells Blood Substit. Immobil. Biotechnol. 1994; 22(3)889–893
- Yu W. P., Chang T. M.S. Submicron polymer membrane hemoglobin nanocapsules as potential blood substitutes: preparation and characterization. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996; 24(3)169–183
- Zambaux M. F., Bonneaux F., Gref R., Dellacherie E., Vigneron C. Preparation and characterization of protein C‐loaded PLA nanoparticles. J. Contr. Rel. 1999; 60: 179–188