Abstract
Chitosan microcarriers were prepared using oxidized lactose as a cross‐linking agent which was oxidized by sodium periodate. The effect of amount of oxidized lactose on the preparation of chitosan microcarriers was studied and optimized. Rat hepatocytes cultivated on chitosan microcarriers cross‐linked by oxidized lactose retained the spherical shape as they have in vivo. Liver‐specific functions such as albumin secretion and glucose metabolism were stably maintained for seven days. The metabolic activities of hepatocytes cultured on the oxidized lactose cross‐linked chitosan microcarriers were higher than those of hepatocytes on chitosan microcarrers cross‐linked by glutaraldehyde and on cytodex 3. The results suggest that oxidized lactose could be an interesting cross‐linking agent for chitosan thus reducing the toxic side effects caused by using glutaraldehyde.
Introduction
The development of cell based organs and tissue engineering devices such as hybrid artificial liver requires that a large number of cells be cultured for the replacement of the damaged tissue (Hayes and Lee, [Citation2001]; Kaneko et al., [Citation1998]). Since its introduction in 1967, microcarriers culture has been applied successfully in growing primary cells and cell lines with the advantage of attaining high cell density (Kong et al., [Citation1999]; Schrimpf and Friedl, [Citation1993]). Spheroid culture is another promising hepatocyte culture method to enhance the cell density and metabolic activities (Hiroshi and Masashi, [Citation1999]; Wu et al., [Citation1996]) Freshly isolated primary hepatocytes can be cultured into three‐dimensional, tightly packed, multicellular aggregates or spheroids. These specialized cell structures have been observed to exhibit enhanced liver‐specific activities and prolonged differentiated state compared to cells that were maintained as a monolayer. Cells in spheroids appear to mimic the morphology and ultrastructure of those in vivo liver lobule (Wu et al., [Citation1996]). The ability of these hepatocytes to organized into three‐dimensional structure is hypothesized to contribute to their enhanced liver‐specific activities. It will greatly enhance the cell density and liver‐specific activities by combing microcarrier culture with spheroid culture.
Chitosan is considered to be a very promising biopolymer for various biomedical and pharmaceutical used because of its nontoxic and biocompatible nature (Shigemasa and Minami, [Citation1995]). Chitosan microcarriers crosslinked by glutaraldehyde and formaldehyde have been reported (Guibal et al., [Citation1999]; Juang and Wu, [Citation2001]) However, the use of such synthetic cross‐linkers can lead to toxic effects, due to the presence of residual cross‐linking agent. Lactose is readily available in common use in the human diet and is potentially biocompatible. To our knowledge, no oxidized sugars were described as a cross‐linking agent for chitosan microcarriers. So oxidized lactose is a novel non‐toxic cross‐linker which can be used in the preparation of chitosan microcarriers.
The objectives of the present work are 1) the preparation and characterization of cross‐linked chitosan microcarriers by using oxidized lactose and 2) the evaluation of the effect of oxidized lactose cross‐linked chitosan microcarriers in the culture of rat hepatocytes.
Materials and Methods
Materials
Chitosan (90% deacetylated) with a molecular weight of 8.0 × 105 was purchased from Chemical Factory of Yuhuan County Zhejiang Province; Fetal calf serum were obtained from Academy of Military Medical Sciences, Tianjin, China; Collagenase D was purchased from Sigma Chemical Co. Albumin kit and glucose kit were purchased from Beijing Zhongsheng High‐tech Bioengineering Co, P.R.China. Male Wistar rat was purchased from Tianjin Medicine Institute.
Preparation of Microcarriers
Oxidation of Lactose
20 grams of lactose were dissolved in 300ml of distilled water. When the temperature of the solution was reduced to 4°C, 30 grams of sodium m‐periodate was added slowly so that the temperature could be controlled between 10–15°C, then the solution was stirred in the dark. After 18h, the solution was cooled to 4°C and sodium hydrogen sulfite was added in order to reduce the unreacted sodium m‐periodate. The pH was adjusted to 5 and the solution was kept at 4°C for 24h. The precipitate was removed and barium chloride was added into the filtrate to remove the sulfate ion. Then the solution was filtrated and stored at 4°C.
Preparation of Microcarriers
Chitosan was dissolved in 3% acetic acid. A certain amount of oxidized lactose was added to 2.5% w/v chitosan solution. Then 50ml of this pre‐cross‐linked chitosan solution was added to 200ml liquid paraffin containing 2% w/w span 80 at 40°C. The mixture was mechanically stirred at 600 rpm for 30 min. Then a suitable amount of oxidized lactose was incorporated into the emulsion and maintained for 1h. The system was adjusted to alkaline by 6% NaOH solution and kept for another 2h. When the reaction was completed, the obtained microcarriers were washed with petroleum ether, alcohol, hydrochloride acid, sodium hydroxide and distilled water successively to elute the liquid paraffin and the excessive oxidized lactose. Light yellow microcarriers were obtained.
Reduction of Microcarriers
30mM sodium acetate was added to chitosan microcarriers and the reduction of the Schiff's base was performed in the presence of sodium borohydride overnight at room temperature under dark condition.
Characterization of Microcarriers
Measurement of the Density
A sample of chitosan microcarriers was submerged in 0.9% sodium chloride solution, and then the weight(m) and volume(v) of microcarriers were accurately measured. The density(ρ)of the microcarriers was calculated by using the following equation:
Determination of the Water Content
A sample of swollen beads was accurately weighed. Then it was dried at 40°C to a constant weight. The water content of the microcarrier was calculated according to the formula:
Where W1 and W2 are the weight of the wet and dried beads, respectively.
Cell Isolation and Culture
Cell Isolation
Liver cells were isolated from male Wistar rat weighting from 180–220g by perfusion of the liver with 0.05% collagenase according to the method of Seglen, ([Citation1976]). The total liver cell suspension was centrifuged at 400rpm for 2 mins three times. The isolated hepatocytes were suspended at 1 × 106 cells per ml in Williams medium E containing 10% calf serum, 0.292 mg/ml L‐Gln, 0.2 units/ml insulin and 80U/ml gentamycin sulfate. Hepatocytes with more than 90% viability, assessed by trypan blue exclusion, were used for the experiments.
Cell Culture Conditions
Isolated hepatocytes were seeded at a density of 5 × 105 cells/ml onto microcarriers in the 6‐well polystyrene culture plates. After 6 hrs, the medium and unattached hepatocytes were removed and fresh medium was added. The medium was changed every day thereafter. The microcarriers and the cell morphology were observed via light and scanning electron microscope(Hitachi X‐650, Japan).
Determination of Liver Specific Functions
Liver specific functions were measured in terms of albumin secretion and glucose metabolism. The albumin concentration in the culture medium was measured using a commercial albumin kit (Beijing Zhongsheng High‐tech Bioengineering Company, P.R.China). And the concentration of glucose was measured using a commercial glucose kit (Beijing Zhongsheng High‐tech Bioengineering Company, P.R. China).
Results and Discussion
Preparation of Chitosan Microcarriers (CMs)
Some characters of microcarriers are important in cell culture such as density, strength, elasticity and water content. shows the effect of the amount of oxidized lactose on the physical properties of the CMs.
Table 1. The Effect of the Amount of Oxidized Lactose on the Properties of Chitosan Microcarriers(CMs).
The amount of oxidized lactose is an important factor influencing the strength, elasticity and density of CMs. As the amount of oxidized lactose increased, CMs became more rigid and less elastic. And the density of CMs also increased. Microcarriers in cell culture should have a proper density, so that the microcarriers can be suspended when they are stirred gently in the culture medium. Elasticity is another important character of microcarriers in cell culture. Elastic microcarrier can cause less damage to cells. So when the amount of pre‐cross‐linker was 6 ml and the supplemented oxidized lactose was 20 ml, CMs with good properties could be prepared.
Morphology of Hepatocytes
The morphology of CMs and hepatocytes attached on the microcarriers was studied by SEM analysis (). CMs showed good spherical geometry and smooth surface. As shown in , hepatocytes attached on the CMs retained spherical shape and aggregated into three‐dimensional spheroids.
Figure 1. SEM of rat hepatocytes on_CMs after three days culture (a) on CMs cross‐linked by glutaraldehyde (b) on CMs cross‐linked by oxidized lactose.
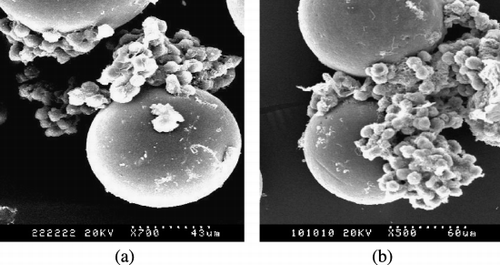
Within spheroids cells appeared to be in close contact with each other which indicated the presence of cell‐cell communication and there were many microvilli on the surface of hepatocytes indicating healthy cells. Bigger spheroids and higher cell density are found in than in , which demonstrate that CMs cross‐linked by oxidized lactose is more propitious to the attachment of hepatocytes than CMs cross‐linked by glutaraldehyde. The results also show that chitosan is a biocompatible material for hepatocytes culture.
Determination of Albumin Secretion and Glucose Metabolism
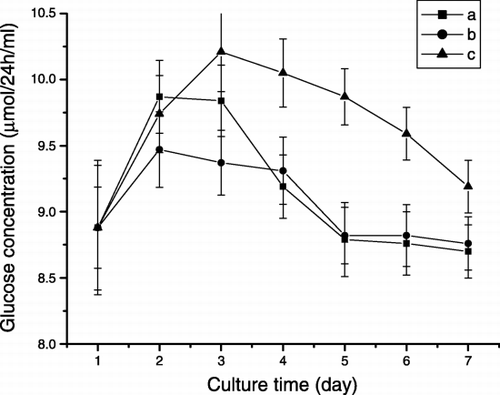
The ability of hepatocytes cultivated on microcarriers to perform liver‐specific functions was evaluated. Albumin secretion and glucose metabolic activities are depicted in and , respectively.
Figure 2. Albumin secretion of hepatocytes cultured on the CMs (a) Cytodex 3 (b) CMs cross‐linked by glutaraldehyde (c) CMs cross‐linked by oxidezed lactose.
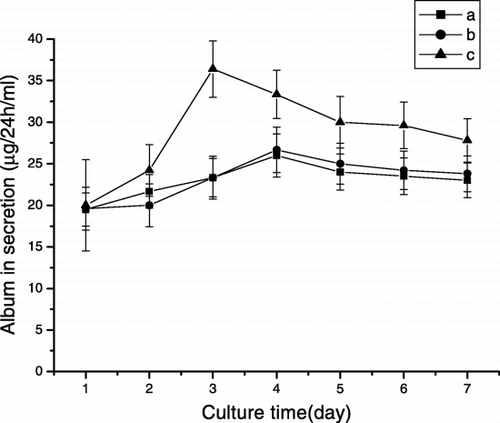
Figure 3. Glucose metabolism of hepatocytes cultured on CMs (a) Cytodex 3 (b) CMs cross‐linked by glutaraldehyde (c) CMs cross‐linked by oxidized lactose.
Albumin Secretion
shows the activity of albumin secretion. On the third day of culture, the albumin secretion rate of hepatocytes cultured on the CMs cross‐linked by oxidized lactose reached its optimal value (36.4µg/24h/ml) and was much higher than that on the CMs cross‐linked by glutaraldehyde and on cytodex 3. It remained stable during 7 days of culture period. On the other hand, the highest activity of hepatocytes cultured on the glutaraldehyde cross‐linked CMs was only 26.7µg/24h/ml. The results demonstrate that oxidized lactose is a biocompatible cross‐linker.
Glucose Metabolism
shows the activity of glucose metabolism. Generally, the glucose metabolic activity was maintained over the course of one week. The metabolic rate of all two cultures continuously decreased during the last 4 days. During 7 days of culture period, the activity of hepatocytes cultured on the CMs cross‐linked by oxidized lactose was significantly higher than that on the CMs cross‐linked by glutaraldehyde and on Cytodex 3. So CMs cross‐linked by oxidized lactose are promising scaffolds for hepatocyte culture and the use of oxidized lactose could be an interesting method to cross‐link chitosan thereby reducing the risk of toxic effect due to the use of synthetic cross‐linkers. Since lactose may be partly oxidized, there were some residual lactose groups remained. According to the literature, lactose could enhance the metabolic activities of hepatocytes (Annie et al., [Citation1996]), so the residual lactose groups that contributed to the enhancement of metabolic abilities.
Conclusion
The results showed that hepatocyes cultured on CMs cross‐linked by oxidized lactose had higher metabolic abilities than those on CMs cross‐linked by glutaraldehyde and Cytodex 3.
Microcarrier culture and spheroid culture are two well‐known cell culture methods. By combining microcarrier culture with spheroids culture, enhanced results were obtained, which could be beneficial to the design and application of hybrid artificial liver support system in the future.
Acknowledgment
The research work was supported by the National Natural Science Foundation of China (Granted No3007022).
References
- Annie T. G., Hungnan L., Joanne Z. Engineering of a sugar‐derivatized porous network for hepatocytes culture. Biomaterials 1996; 17: 387–393
- Guibal E., Milot C., Eterradossi O. Study of molybdate ion sorption on chitosan gel beads by different spectrometric analyses. Int. J. Biol. Macromol. 1999; 24(1)49–59
- Hayes P. C., Lee A. What progress with artificial livers?. Lancet 2001; 358(9290)1286–1287
- Hiroshi M., Masashi H. Formation of cylindrical multicellular aggregate(cylindroid) and expression of liver specific functions of primary rat hepatocytes. Cytotechnology 1999; 31: 69–75
- Juang R.‐S., Wu F.‐C. Solute adsorption and enzyme immobilization on chitosan beads prepared from shrimp shell wastes. Bioresour. Technol. 2001; 80(3)187–193
- Kaneko M., Fukuda J., Ijima H. Development of hybrid artificial liver support system using spheroid culture and application to warm ischemic liver failure in dog and pig as a preclinical test. Mater. Sci. Eng., C, Biomim. 1998; 6(4)245–248
- Kong M. D., Chen M., Gentz R., Zhang J. Cell growth and protein formation on various microcarriers. Cytotechnology 1999; 29: 151–158
- Schrimpf G., Friedl P. Growth of human vascular endothelial cells on various types of microcarriers. Cytotechnology 1993; 13(2)89–98
- Seglen P. O. Preparation of isolated rat liver cells. Method Cell Biol. 1976; 13: 29–83
- Shigemasa Y., Minami S. Application of chitin and chitosan for biomaterials. Biotechnol. Genet Eng. Rev. 1995; 13: 383–420
- Wu F. J., Friend J. R., Hsiao C. C., Zilliox M. J., Ko W.‐J., Cerra F. B., Hu W.‐S. Efficient assembly of rat hepatocyte spheroids tissue engineering applications. Biotechnol. Bioeng. 1996; 50: 404–415