Abstract
Cephalosporins are usually produced semisynthetically from Cephalosporin‐C, which is exclusively produced by Cephalosporium acremonium. Free cell studies for the production of Cephalosporin‐C had some limitation such as pulpy growth of fungus causing an appreciable rise in the broth viscosity affecting the transfer of oxygen and other nutrients into the cells. High cell concentrations cannot be maintained because of wash out phenomenon at high dilution rates. The whole cell immobilization technique is a potentially important process for Cephalosporin‐C biosynthesis, where increase cell densities were maintained and broth‐handling problems were reduced. Cephalosporin‐C fermentation is a highly aerobic process. The symbiotic relationship of Cephalosporium acremonium and Chlorella pyrenoidosa has been used to increase oxygen transfer rate to the fungi by co‐immobilizing it with algae. Co immobilization of whole cells of fungus and algae were carried out in different immobilizing agents and the systems were coated with polyacrylamide resin of pharmaceutical grade to overcome the problems of leakage. The operational stability of immobilized systems in a packed bed reactor was also studied.
Introduction
Cephalosprins, important β‐locate antibiotics, more resistant to β‐lactamases than penicillins are usually produced semisynthetically from Cephalosporin‐C (CPC) which is exclusively produced by fermentation (Bayer et al., [Citation1990]). It has a relatively weak antibacterial activity, but the modification of its side chain generates semisynthetic Cephalosprins having diversified antibacterial activity. The cephalosporin‐C fermentation is a highly aerobic process requiring high volumes of air to maintain its metabolic function to last the entire fermentation period (Mahapatra, [Citation1998]). Oxygen, because of its low solubility in aqueous phase, makes the problem were difficult. Several authors have followed different techniques for increasing the oxygen solubility in the fermentation broth (Aldercruetz and Mattiasson, [Citation1982]; Aldercruetz et al., [Citation1982]; Khang et al., [Citation1988]), but none can compare the in‐situ supply of oxygen where mixed culture techniques are followed. In such systems specific metabolic properties of two different organisms have to be compatible. In a simple gel bead, there is a combination and utilization of specific metabolic properties of two different microorganism. Thus, to generate oxygen in‐situ, oxygen consuming organisms (mold) are co‐immobilized with oxygen producing organisms (algae). Algae are of great importance because of their ability to produce oxygen and derive cell carbon from CO2. The gaseous exchange takes place in the cellular microenvironment in which case both the organisms have to be symbionts.
The in‐situ production of oxygen has been found to be an easier, cheaper and non‐toxic method for supplying oxygen to aerobic process. Various immobilization methods have been studied for the production of metabolites (Doran and Bailey, [Citation1986]; El‐Sayed and Rehm, [Citation1986]; Morikawa et al., [Citation1979]). The major problems, which arise with whole cell immobilization, are mass transfer limitation, flow rate optimization, leakage of cells and recycling of the output. The present investigation describes the co‐immobilization of whole cells of Cephalosporium acremonium and Chlorella pyrenoidosa using various immobilizing agents, optimization of the process and to stabilize the beads by coating with a suitable resin, thus reducing the leakage of cells.
Materials and Methods
Organisms
Cephalosporium acremonium NCIM 1069 used for the production of Cephalosporin‐C and Chlorella pyrenoidosa NCIM 2738 used for the co‐culture studies, were obtained from the National Chemical Laboratory, Pune, India. Stock slants of fungus were maintained on potato‐dextrose‐agar after incubation for 5–8 days at 28°C, while the algae was maintained on agar slants which contain in one liter, magnesium sulphate (2.5 g), potassium nitrate (1.25 g), potassium dihydrogen orthophosphate (1.25 g), peptone (2.0 g), glucose (5.0 g), ferric sulphate (1.0 ml) of 4 gL− 1 solution and agar (30 g). The pH was adjusted to 4.5 and incubated at 28°C under illuminating orbital shaker for 5–7 days.
Inoculum
For both fungi and algae, the surface growth from their seven days old slant was suspended in 5.0 ml sterilized distilled water and used for the inoculation of seed media.
Culture Medium and Conditions
Seed and production media composition as described by Kundu et al. ([Citation1992]) (Kundu et al., [Citation1992]) were used for the production of cephalosporin‐C in monoculture studies. Seed culture medium for algae contained in one litre, glucose (5.0 g), potassium nitrate (5.0 g), magnesium sulphate (2.5 g), potassium dihydrogen orthophosphate (1.25 g), ferrous sulphate (0.003 g), ethylene diaminotetra‐acetic acid (EDTA) (0.037 g) and Arnon's A4 solution (1.0 ml) which was autoclaved separately (Mahapatra, [Citation1998]). The pH of the seed medium was adjusted to 5.5. The Arnon's solution contained in one litre, ammonium vanadium trioxide (0.02296 g), manganese chloride (1.81 g) and zinc sulphate (0.222 g). The algae was cultivated at 28°C in an orbital shaker at 300 rpm for 5–7 days with intermittent light supply.
The production medium for co‐culture studies contained in one litre, magnesium sulphate (2.5 g), sodium nitrate (1.0 g), sodium bicarbonate (1.0 g), potassium dihydrogen orthophosphate (1.25 g). DL‐methionine (3.0 g), sucrose (10.0 g) and trace metal solution (1.0 g) which was autoclaved separately. The pH was adjusted to 7.3–7.5 with sodium hydroxide. The trace metal solution contained in one litre, ferrous sulphate (4.0 g), boric acid (2.9 g), magnesium chloride (0.02 g). These were mixed aseptically and fermentation was carried out at 28°C under illumination.
Immobilization Methods
Whole cell immobilization was followed for confining the fungal and algal cells using various immobilizing agents.
Immobilization in Alginate
Actively growing cells from a three day old seed culture were centrifuged at 3000 × g for 10 min and supernatant discarded. Concentrated seed of 25 g was mixed with 60 ml of sterile sodium alginate solution (2 g in 90 ml distilled water) and added drop wise through a needle (no. 18 gauge) to a stirred solution of 2% w/v calcium chloride (or strontium chloride or barium chloride). The beads so formed were cured for 1.5 h at 30°C temperature, washed with sterile distilled water and resuspended in 1.0% sterile glucose solution till further studies. Different proportion of fungus and algae were entrapped in this way. For resin coated systems, polyacrylamide resin solution9 was used either ffor spraying (200 sec contact time and 200 sec drying time) or immersing (30 sec contact time and 200 drying time) the alginate beads, Polyethylene glycol (600) was added proportionately to prevent films being brittle.
Immobilization in Bagasse
Bagasse (agriculture residue) was thoroughly washed and dried for 4 days. Its pith was chopped into 2 cm pieces and sterilized. About 2 g of such carrier was suspended in 100 mL of sterile production medium containing concentrated seed culture and incubated in an orbital shaker at 28°C for one day at 60 rpm and for three days at 100 rpm. It was washed with phosphate buffer (0.1 M, pH 7.0) to remove the loosely bound cells before being packed into the presterilized packed bed reactor. It was left to rest for 2–3 h in 1.0% glucose solution in the reactor.
Immobilization in Silk Sachet
Silk sachets (2 cm × 2 cm) (katan silk,Varanasi, India) (Mahapatra, [Citation1998]) were washed, dried and sterilized. Concentrated seed culture (Kundu et al., [Citation1992]) was transferred to these carriers aseptically. These were washed with 0.1 M phosphate buffer (pH 7.0) to remove any loosely bound cells. They were then loaded on to the pre‐sterilized reactor aseptically. Different ratios of fungus to algae were taken and sachets were prepared accordingly. Coating with resin was carried out in the same way as described in resin coated alginate bead preparation.
Packed Bed Bioreactor
Co‐immobilized cells of fungi and algae were packed in the presterilized glass reactor (height 70.3 cm and diameter 3.8 cm). The feed was passed through the bottom of the reactor at varying flow rates by a pre‐calibrated peristaltic pump and the product collected from the top of the reactor was assayed for antibiotic content. The temperature inside the reactor was maintained by passing water having appropriate temperature through the external jacket. The operational stability of the reactor was investigated by continuously passing the feed at 28°C for 120 h and measuring the degree of conversion at various time intervals. The reactor operation was carried out in continuous mode.
Analysis
The method of Boxer and Everett ([Citation1949]) (Boxer and Everett, [Citation1949]) was used for Cephalosporin‐C estimation. Total reducing sugar in the filtrate was determined by DNS method (Miller, [Citation1959]) and sucrose was estimated similarly after prior hydrolysis. The dry cell weight was estimated by the method of Millis et al. ([Citation1963]) (Millis et al., [Citation1963]). Dissolved oxygen measurement was made using a pre‐calibrated DO probe (Bioengineering AG).
Results and Discussion
Selection of Immobilizing Agents
Bagasse, silk sachet, alginate and alginate coated with polyacrylamide resin were used for the co‐immobilization studies of C. acremonium and C. pyrenoidosa. Both the microorganisms had low adsorption on bagasse. Algal cells because of their unicellular microscopic structure escaped through the silk sachets (Joshi, [Citation1991]). The leakage of algal cells was higher with uncoated alginate beads in comparison to alginate beads coated with polyacrylamide resin.
Effect of Cephalosporin‐C on the Growth of Algae
Different concentration of Cephalosporin‐C solution (2.0 mL) was added to algal culture in fresh nutrient and incubated at 28°C under light for 5–7 days. Algal cell growth increased with incubation time (observed upto 72 h) irrespective of the concentration of Cephalosporin‐C (even at 600 mg/mL). If Cephalosporin‐C limits the growth of alga, their level would have decreased with time of incubation. Algal cell growth was not inhibited by Cephalosporin‐C which is the final metabolite of C. Acremonium indicating a possibility of non‐inhibitory relationship between algae and fungus ().
Table 1. Algal Cell Growth at Different Concentration of Cephalosporin‐C
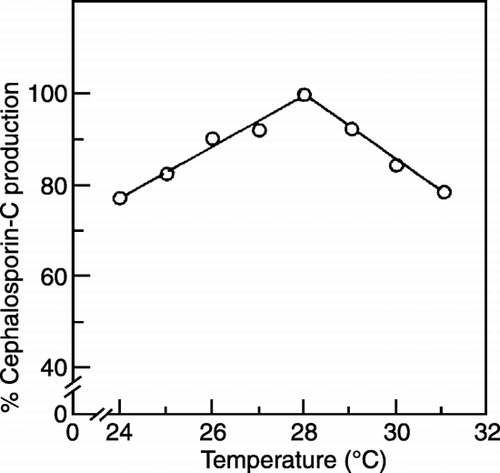
Effect of Temperature on Cephalosporin‐C Production by Co‐immobilization
Immobilized monoculture studies showed fungal cells to be growing well at 28°C and the optimum temperature for the synthesis of CPC was 28°C (Mahapatra, [Citation1998]). Algal cells were found to be growing well in between 25°C and 28°C. The temperature for the production of CPC using co‐immobilized cells of Ca‐alginate beads are found to be 28°C and above that the yield of Cephalosporin‐C decreased due to the inactivation of enzymes involved in the Cephalosporin‐C biosynthesis (). Hence, the co‐immobilization studies were carried out at 28°C.
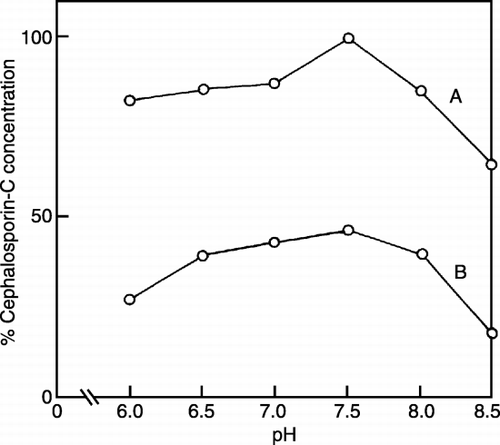
Effect of pH on Cephalosporin‐C Production by Co‐immobilization
The effect of pH on the production of CPC was carried out in two different sets of experiments (). One set of experiments had carbonate as one of the substrates and the other set did not have any carbonate. It was observed that the change in CPC production was rather insignificant between pH 6.0–7.0 and very low at pH 8.5 when carbonate was used as substrate for algae. The decreased production of CPC at higher pH may be due to markedly decreased activity of fungus at pH 8.0 and above. In the absence of carbonate, the production of CPC was observed to be fairly constant in the pH range between 6.5 and 8.0, showing an optimum pH of 7.5. However, the production of CPC was lower than the production obtained in the presence of carbonate. The optimum pH for CPC production in both experiments was found to be 7.5.
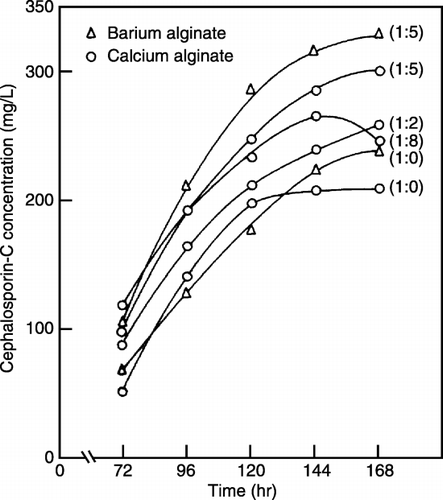
Effect of Ratio of Fungus to Algae on Cephalosporin‐C Production by Co‐immobilization
The production of Cephalosporin‐C was increased as the ratio of cell dry weight of fungus and algae was increased from no algae to a fungus to algae ratio of 1:5. It was observed that the yield of CPC using co‐immobilized cells becomes 1.43 times of that of immobilized cells of C. acremonium alone after 168 h of fermentation. However, the antibiotic concentration decreased at a fungal to algal ratio of 1:8 (). This may be attributed due to the non‐availability of enough substrates for the growth of fungus as a result of competetion between algae and fungus for the same substrate. Another probable reason may be molecular oxygen toxicity to the fungus due to increased production of oxygen by algae, C. pyrenoidosa.
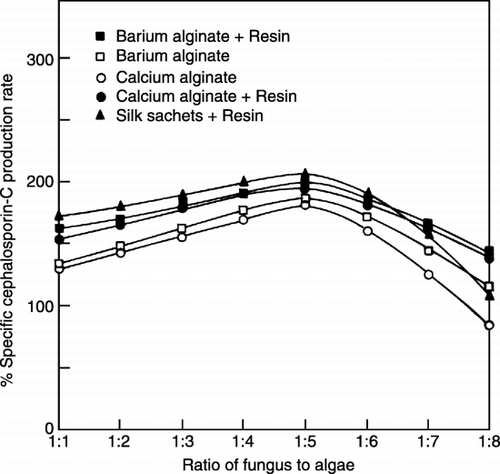
Effect of Coating on Immobilization Studies
The reactor was packed with different ratios of fungus to algae and operated at a flow rate of 30 mL/h (). It was found that specific production rate was better with an optimum fungus to algae ratio of 1:5, and in both the systems where coated and uncoated beads were used respectively showed decreased production at higher ratios of fungus to algae. Same observations were noted for the system using coated silk sachets. Increased cell loading reduces the oxygen diffusion, and therefore, mass transfer resistances increased. Lesser amount of substrates reach to the core of beads and lesser light intensity is available thereby retarding the growth of the algae. Hence, Cephalosporin‐C production decreases. Coated beads gave better specific production rate than the uncoated ones since, the acrylic resin [(Eudragt RL 100)/ethanol (96% v/v)] (Mahapatra, [Citation1998]) solution gave a highly permeable stable matrix on the bead wall and an increase in diffusion coefficient of substrates and products was observed due to the dehydration effect of 96% ethanol on the matrix (Ruggeri et al., [Citation1991]). Amount of oxygen produced by higher proportions of algal cells in beads might have caused undue tension on the bead wall resulting in deterioration of the matrix in uncoated beads.
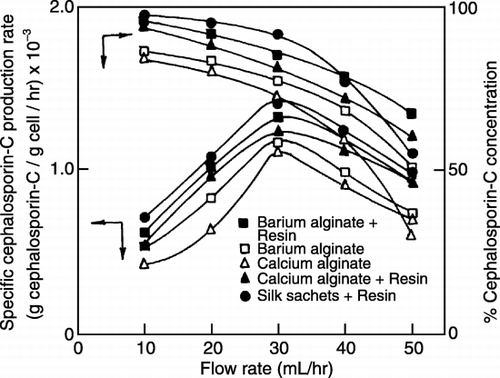
Effect of Flow Rate on Cephalosporin‐C Production by Co‐immobilization
The flow rate of the feed in the packed bed reactor was found to be optimum at 30 mL/h for CPC production in co‐immobilized systems (). The antibiotic production increased with a decrease in flow rate because of longer residence time, while beyond a certain flow rate the antibiotic production fell steeply suggesting a critical residence time upto which the production was effective. Nominal increase in production at lower feed velocities indicated the resistance offered by the immobilization systems. Specific antibiotic production rate was higher with silk sachets coated with resin ().
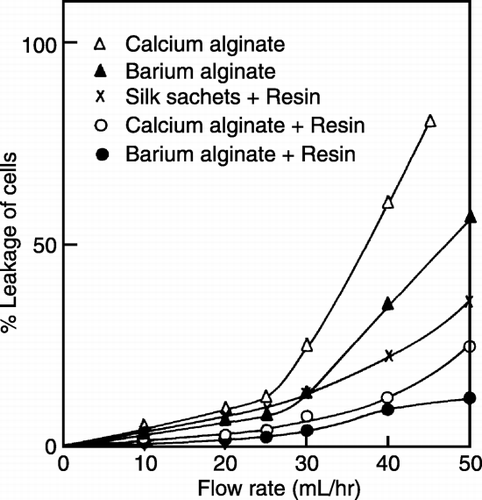
Leakage of Cells
Uncoated alginate beads exhibited more cell leakage than the coated ones and leakage was observed at all flow rates and increased steeply with increase in flow rate (). It was observed that systems having resin coated beads/silk sachets showed better stability than the system using uncoated beads. Resin coated beads/silk sachets because of their stable matrix caused low cell leakage leading to higher production of CPC. The leakage was lower for barium alginate beads showing these are more stable than the calcium alginate beads. Biomass activity index (the percentage of specific Cephalosporin‐C production with respect to uncoated alginate beads) of immobilized systems are shown in .
Table 2. Effect of Coating on Biomass Activity Index
From this table, it is clear that spraying had a negative influence on the activity of beads. This could be due to longer contact time during spraying methodology. Hence, immersion method was followed for coating alginate beads and silk sachets before loading into the reactor for coculture studies.
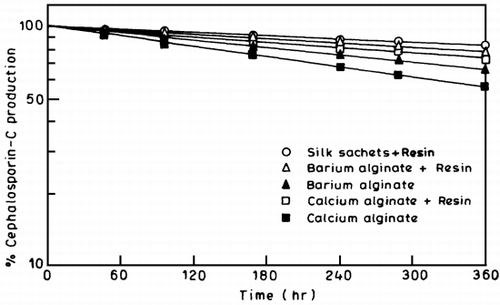
Operational Stability of Immobilized Systems
The packed bed reactor was operated under steady state conditions for all the immobilized systems. Half lives of all the immobilized systems were computed and represented in . The progress of continuous production of CPC is shown in as semi logarithmic plot. The decline in productivity could be due to a decrease in immobilized cell viability. There could be a gradual deterioration of carrier matrix eventually resulting in the loss of cell loading from the carrier. The resin coated coculture systems responded well and were more stable during the operation than the uncoated systems. Among the alginate systems, barium showed better stability than the calcium since the strength of the complex increases along the series Ca+ 2, Sr+ 2 and Ba+ 2 (Park and Khang, [Citation1995]) however under the conditions used, the system using silk as the carrier was most stable over a longer period of time.
Table 3. Half‐Lives of Various Co‐immobilized Systems
Conclusions
Biosynthesis of CPC by coimmobilized systems of Cephalosporium acremonium and Chlorella pyrenoidosa using various modes of immobilization was studied at the optimal conditions of pH, temperature, ratio of fungi to algae and flow rate etc. In‐situ generation of oxygen led to an increase of antibiotic production. A favourable microenvironment was created during co‐immobilization of fungus and algae, indicating improved performance of the reactor. Packed bed studies were carried out to assess the stability of the various co‐immobilized systems. It indicated that the resin coated beads were more stable than the uncoated beads. Hence, it can be efficiently run in a continuous mode at a flow rate of 30 mL/h to obtain the maximum specific antibiotic production.
Acknowledgments
Dr. Subir Kundu is grateful to All India Council for Technical Education and University Grants Commission for the financial assistance received as research grant. Dr. Amulya Chandra Mahapatra and Dr. Vinod Kumar Nigam gratefully acknowledge the financial assistances provided by University Grants Commission and Council of Scientific and Industrial Research, India, respectively as research fellowship during entire period of their research work.
Nomenclature
| CPC: | = | Cephalosporin‐C |
| DO: | = | Dissolved oxygen (%) |
| X: | = | Dry cell mass (g/L) |
| T: | = | Time (h) |
| T1/2: | = | Half‐life |
References
- Aldercruetz P., Mattiasson B. Oxygen supply to immobilized cells. 1. Oxygen production by immobilized Chlorella pyrenoidosa. Enzyme Microb. Technol. 1982; 4: 332–336
- Aldercruetz P., Holst O., Mattiasson B. Oxygen supply to immobilized cells. 2. Studies on a coimmobilized algae‐bacteria preparation with in‐situ oxygen generation. Enzyme Microb. Technol. 1982; 4: 395–400
- Bayer T., Herold T., Holzhauer K., Zhou W., Schugerl K. Comparison of Cephalosporin‐C production in stirred tank and air lift tower loop reactors. Ann. N.Y. Acad. Sci. 1990; 589: 665–669
- Boxer G. E., Everett P. Colorimetric determination of benzylpenicillin (colorimetric determination of total penicillins). Anal. Chem. 1949; 21: 670–673
- Doran P. M., Bailey J. E. Effects of immobilization on growth, fermentation properties and macromolecular composition of Saccharomyces cerevisiae attached to gelatin. Biotechnol. Bioeng. 1986; 28: 73–87
- El‐Sayed A.‐H. M.M., Rehm H. J. Morphology of Penicillium chrysogenum strains immobilized in calcium alginate beads and used in penicillin fermentation. Appl. Microbiol. Biotechnol. 1986; 24: 89–94
- Joshi J. Microbial production of Cephalosporin‐C using co‐immobilized cells of C. acremonium and C. pyrenoidosa. M. Tech. Project Report. School of Biochemical Engineering, Institute of Technology, Banaras Hindu University, VaranasiIndia 1991
- Khang Y.‐H., Shankar H., Senotore F. Enhanced β‐lactam antibiotic production by coimmobilization of fungus and alga. Biotechnol. Lett. 1988; 10: 867–872
- Kundu S., Mahapatra A. C., Srivastava P., Kundu K. Studies on Cephalosporin‐C production using immobilized cells of Cephalosporium acremonium in packed bed reactor. Process Biochem. 1992; 27: 347–350
- Mahapatra A. C. Studies on microbial production of Cephalosporin‐C using different processing strategies. Ph.D. Thesis. School of Biochemical Engineering, Institute of Technology, Banaras Hindu University, VaranasiIndia 1998
- Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959; 31: 426–428
- Millis N. F., Trumphy B. H., Palmer M. B. The effect of lipids on citric acid production by an Aspergillus niger mutant. J. Gen. Microbiol. 1963; 30: 367–370
- Morikawa Y., Karube I., Suzuki S. Penicillin G production by immobilized whole cells of Penicillium chrysogenum. Biotechnol. Bioeng. 1979; 21: 261–270
- Park H.‐J., Khang Y.‐H. Production of Cephalosporin‐C by immobilized Cephalosporium acremonium in polyethyleneimine modified barium alginate. Enzyme Microb. Technol. 1995; 17: 408–412
- Ruggeri B., Sassi G., Specchia V., Bosco F., Marzona M. Alginate beads coated with polyacrylamide resin: potential as a biocatalyst. Process Biochem. 1991; 26: 331–335