Abstract
Optimising microencapsulation technology towards the effective clinical transplantation has created the need for highly biocompatible alginates. Therefore, in this study the biocompatibility of different beads prepared from alginates with varying average molecular weight was examined. In some experiments the beads were covered with a multilayer membrane surrounded by an alginate layer. First of all, we found that beads made of a lower weight average alginate elicted a much stronger fibrotic response compared to beads made of a higher weight average alginate (LV‐alginate > MV‐alginate). The results were confirmed by the observation that the extent of tissue fibrosis was significantly increased in multilayer capsules made of an alginate with a lower weight average (core and surface LV‐alginate, Mw 0.7–1*106 g/mol, viscosity of a 0.1% solution 1–2.5 mPa s− 1) compared to multilayer capsules made of an alginate with a higher weight average (core and surface MV‐alginate; Mw 1.2–1.3*106 g/mol, viscosity of a 0.1% solution 5–7 mPa s− 1). It should be stressed, that the pro‐fibrotic effect of the LV‐alginate alginate in the core was only partially reversed by a MV‐alginate on the surface of the multilayer capsules. On the basis of the raised data, it can be assumed that the molecular weight average of the alginates have an decisive effect on the biocompatibility. Therefore, it seems to be recommendable to reduce the low molecular weight fractions of the alginate during the purification process to improve the biocompatibility.
Introduction
Transplantation of therapeutic cells, e.g. pancreatic islets, is a promising strategy for the treatment of various hormone deficient and neurodegenerative disorders (Mach et al., [Citation2003]). Host reactions against allogeneic or xenogeneic cells is postulated to be eliminated by encapsulation, thus preventing interactions of the encapsulated cells with the components of the immune system (Zimmermann et al., [Citation2000], Citation[in press]). Recent pilot clinical trials have shown the feasibility of alginate‐based microcapsules for immunoisolated transplantation (Hasse et al., [Citation1997]). Transplantation of Ba2 +‐alginate‐encapsulated allogeneic parathyroid tissue into the forearm muscles of two patients with hypoparathyroidism demonstrated normal levels of parathormon and normocalcemia, shortly after surgery. However, graft failure occurred after about three months. Research during the past five years has shown that the biocompatibility and immuno‐protective properties of the microcapsules is not only influenced by the chemical composition and purity of the material, but also by physical and physicochemical imperfections of the microcapsules. Alginate microcaspules show a high tendency to take up large amounts of water, which causes them to swell. Swelling is most likely one of the main reasons for formation of cracks and microcapsule breakage leading ultimately to a failure of the graft. The prevention of swelling has not only an enormous impact on the mechanical strength, but also on the diffusion properties of the microcapsules. Swelling can be prevented by incorporation of proteins or perfluorocarbons and by removal of the excessive internal divalent cations once the cross‐linking process is completed (Hillgärtner et al., [Citation1999]; Zimmermann et al., [Citation2000]).
Of equal importance for the mechanical strength of the microcapsules seems to be the viscosity of the alginate and thus the average molecular mass of its polymeric guluronic‐ and mannuronic acid chains. In this communication we demonstrate by quantitative image analysis of the fibrotic overgrowth of alginate capsules retrieved after 4‐week transplantation in rats, that the viscosity of the alginate also dictates the chemical biocompatibility of the microcapsules. To this end, homogeneous and multilayered capsule of various compositions were studied by using a medium‐viscosity commercial alginate as well as a low‐viscosity alginate made by autoclaving. The results show that fibrotic overgrowth increases with decreasing viscosity and thus with the average molecular mass independent of the structural configuration of the microcapsule.
Methods and Materials
Alginate
Alginates of medium‐viscosity (MV‐alginate) were supplied by Pronova Biopolymer, Drammen, Norway, (Guluronic acid content 73%). Before use, the alginate solution was sterile‐filtered over 1.2, 0.8, 0.45 and 0.2 µm filters (Schleicher & Schuell). The viscosity of a 0.1% solution was determined to be 5–7 mPa s− 1 by using an AMV‐200 viscosity meter (A. Paar KG, Graz, Austria). Low‐viscosity alginates (LV‐alginate) were made by autoclaving of the dried MV alginate for 20 min at 121°C. These procedures decreased the viscosity of the alginate to 1–2.5 mPa s− 1. For microcapsule formation the viscosity of the different alginate solutions (MV vs. LV) were adjusted to the same working viscosity.
Capsule Preparation
For capsule formation an air driven droplet generator was used (Schneider et al., [Citation2001], Citation[in press]). The alginate droplets were allowed to gel in a 20 mM BaCl2 (adjusted to pH 7,4) for 10 min. Then, the beads were washed three times with physiological saline solution followed by an incubation for 30 minutes in 6 mM Na2SO4 at 37°C and finally incubated for 30 minutes in physiologic saline solution at 37°C. In one set of experiments the capsules were coated with a multilayer membrane as described in detail by Schneider et al. ([Citation2001]). Briefly, the multilayer membrane was formed by suspending the microcapsules in a 0,03% (w/w) polycation solution (polyethylenimine, Fluka, Steinheim, Germany, MW 1.000.000) for 3 min. Excessive polycations were removed by washing the microcapsules with physiological saline solution for 2 min. Then, the microcapsules were re‐suspended in a 0.03% (w/w) polyanion solution (polyacrylacid, Sigma, Steinheim, Germany, MW 60.000) for 3 min and the excessive polyanions were then removed as described before. A polycation membrane was finally formed by re‐suspending the microcapsules in a 0,03% (w/w) polycation solution (polyethylenimine, Fluka, Steinheim, Germany, MW 1.000.000) for 3 min, followed by another washing step. Subsequently, the polycation membrane was covered with an alginate layer by incubation of the microcapsules in a 0.03% alginate solution for 3 min. All procedures were performed under sterile conditions.
The homogeneous Ba2 +‐cross‐linked alginate microcapsules as well as the multilayer capsules exhibited an average diameter of about 450 µm as revealed by light microscopy. Furthermore, the portion of capsules with membrane imperfections was estimated. Microscopical inspection prior to implantation revealed no difference concerning the physical integrity of the capsules of the different experimental groups.
Transplantation
Sprague‐Dawley rats weighing 300–350 g were used as recipients (Central Animal Facility, University of Mainz). In each experiment a volume of 0.5 ml microcapsules (app. 10.000 capsules) was transplanted into one muscle pouch (musculus pectoralis). For each experimental setting a group of n = 7 animals has been transplanted. One month after transplantation capsules were retrieved by excision. Explants were embedded in paraffin and sections were stained with haematoxylin‐eosin (HE) as described elsewhere.
Quantification of Fibrosis as a Marker of Biocompatibility ()
Figure 1. A representative example for objective quantification of microbead induced fibrosis. (A) Selection of fibrotic tissue. (B) Selection of alginate capsules. The histograms in (A) fibrotic tissue and (B) alginate capsules are generated (see methods) and afterwards fibrotic reaction was calculated as fibrotic area per alginate capsule (µm2). (Go to www.dekker.com to view this figure in color.)
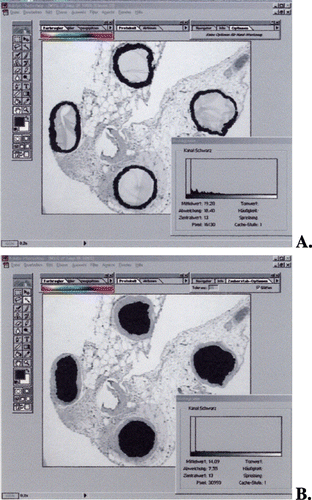
For objective quantification of fibrotic tissue surrounding the alginate capsules, image analysis was performed using the Photoshop software, as described previously (Lehr et al., [Citation1999]). Briefly, using the Magic Wand tool in the Select menu of Photoshop, the cursor was placed on fibrotic tissue. Using the Similar command in the Select menu, the whole fibrotic tissue surrounding the capsules was selected. Using the Fill tool in the Edit menu, the entire selected area was changed to black. Afterwards, the image was transformed to 8‐bit greyscale. An optical density plot of the selected area was generated using the Histogram tool in the Image menu. The Pixel amount corresponding to the fibrotic tissue was recorded. Fibrotic reaction was calculated as pixel of fibrotic tissue/capsule, corresponding to µm2/capsule (dependent on magnification and the resolution of the image). With this technique we are able to measure all the capsules found on the histological sections. To obtain statistically relevant data > 100 capsules per sample were analysed.
Gel Permeation Chromatography (GPC)
The molecular weight distributions of the alginates were determined by high pressure gel permeation chromatography, one of the most common methods to estimate the molecular weight distribution of polymers (Berth, [Citation1992]; Martinsen et al., [Citation1991]; Vandenbossche and Remon, [Citation1993]). The system was equipped with a Gynkotek pump (P 580 A, Gynkotek, Germering, Germany) and three serial columns [HEMA (Bio) 40, (molecular‐weight‐area < 20 kg mol− 1); HEMA (Bio) 1000 (molecular‐weight‐area 20–3000 kg mol− 1), SUPREMA 3000 (molecular‐weight‐area 20–10000 kg mol− 1) [Polymer Standards Service (PSS) Mainz, Germany] followed by a differential refractometer (RI‐71, Gynkotek, Germering, Germany). A 0.05 M NaHCO3/0.1 M NaNO3 aqueous solution was used as eluent. 50 µl of the alginat solution were injected and the flow rate was adjusted to 1.0 ml min− 1. For the different tested alginate samples neither Kuhn‐Mark‐Houwink‐constants are known in the literature nor alginate‐calibration‐standards are free for sale. Therefore, 14 dextran standards (PSS, Mainz, Germany) with molecular weights ranging from 0,18–277 kg mol− 1 were used. Using the WINGPC® Software (Polymer Standards Service (PSS), Mainz, Germany) the number average (Mn) and the weight average (Mw) of the samples were calculated.
Statistical Analysis
Image analysis of the slides histological data was expressed as means ± SEM. Student's t‐test or rank‐sum test were applied to determine statistically significant differences for fibrosis as a marker of biocompatibility of different alginate microcapsules. Differences were considered statistically significant at a p < 0.05 level.
Results
Gel Permeation Chromatography (GPC)( and )
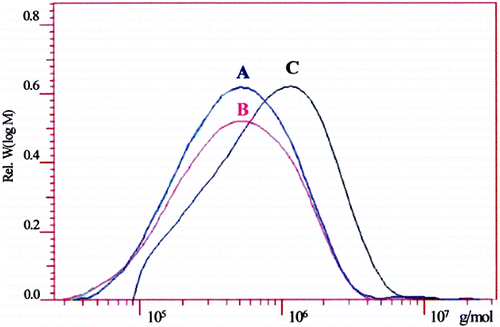
The GPC‐analysis revealed that autoclaving of MV‐alginate causes a significant drop of the weight average (Mw) from 1.2–1.3*106 to 0.7–1.0*106 g mol− 1 for the LV‐alginate. The number average (Mn) decreases also from 5.4*105 to 3.3 105 g mol− 1. Consequently, the polydispersity (D) remains constant, suggesting that autoclaving causes a statistical chain scission of the alginate molecule with an increasing amount of oligosaccharides. Sterile‐filtration of the LV‐alginate leads to a further decrease of the weight average (Mw) from 0.7–1.0*106 to 0.6–0.67*106 g/mol. These data are in agreement with the data of the solutions viscosities (see above).
Table 1. Molecular Weight Distribution of Different Alginate Preparations Performed by Gel Permeation Chromatography. (A) Autoclaved Alginate (LV‐Alginate). (B) Autoclaved Alginate and Afterwards Sterile‐Filtrated (LV*‐Alginate). (C) Sterile‐Filtrated Alginate Solution (MV‐Alginate)
Figure 2. Molecular weight distribution of different alginate preparations performed by gel permeation chromatography. (A) Autoclaved alginate (LV‐alginate). (B) Autoclaved alginate and afterwards sterile‐filtrated (LV*‐alginate). (C) Sterile‐filtrated alginate solution (MV‐alginate). (Go to www.dekker.com to view this figure in color.)
Biocompatibility of Alginate Beads Made of Different Alginate Solutions ()
Figure 3. Extent of tissue fibrosis induced by transplantation of alginate beads made of different alginate solutions into a muscle pouch. Fibrotic reaction was calculated as area of fibrotic tissue/capsule, corresponding to µm2/capsule (dependent on magnification and the resolution of the image). Values are means ± SEM. (Go to www.dekker.com to view this figure in color.)
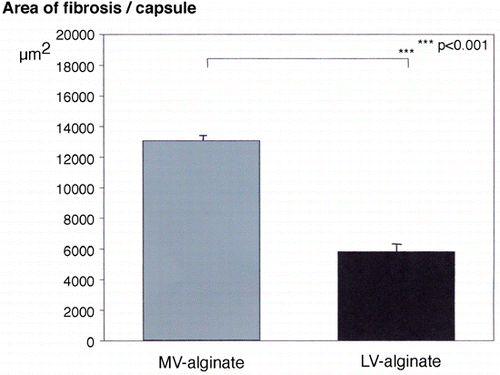
First of all, beads made of an alginate with a lower weight average elicted a much stronger fibrotic response compared to beads made of an alginate with a higher weight average (LV‐alginate: 13080 ± 310 µm2 vs. MV‐alginate: 5824 ± 54 µm2; p < 0.001).
Biocompatibility of Different Multilayer Capsules ()
Figure 4. Extent of tissue fibrosis induced by different empty alginate beads or multilayer microcapsules (A–D) into a muscle pouch. (A) Alginate beads made MV‐alginate without a covering outer membrane. (B) Alginate beads made of MV‐alginate with a PEI‐PAA‐PEI‐alginate (MV‐alginate) covering outer membrane. (C) Alginate beads made of LV‐alginate with a PEI‐PAA‐PEI‐alginate (MV‐alginate) covering membrane. (D) Alginate beads made of LV‐alginate with a PEI‐PAA‐PEI‐alginate (LV‐alginate) covering membrane. Fibrotic reaction was calculated as area of fibrotic tissue/capsule, corresponding to µm2/capsule (dependent on magnification and the resolution of the image). Values are means ± SEM. No statistical differences were found between the values of group A vs. B. (Go to www.dekker.com to view this figure in color.)
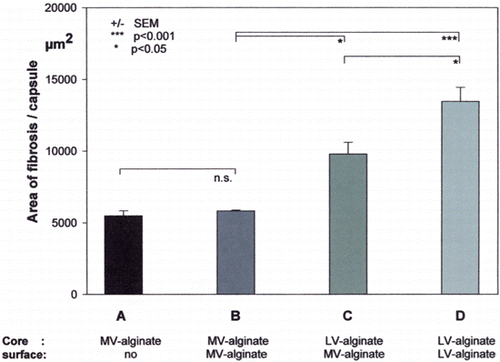
We quantified the extent of tissue fibrosis induced by transplantation of different multilayer capsules into a muscle pouch. We found no difference in the extent of tissue fibrosis between alginate beads without a covering membrane and those covered with an additional multilayer membrane. In contrast to this, the extent of tissue fibrosis was significantly increased in multilayer capsules made of an alginate with a lower weight average (core and surface LV‐alginate) compared to multilayer capsules made of an alginate with a higher weight average (core and surface MV‐alginate). The pro‐fibrotic effect of the LV‐alginate core was only partially reversed by an alginate with a higher weight average (MV‐alginate) on the surface of the multilayer capsules.
Discussion
The present study indicates that the biocompatibility of alginate beads increases with the weight average (Mw) of the used alginate solution. Beads made of an alginate (LV) with a weight average of 0.7–1.0*106 g/mol elicited a much stronger fibrotic response compared to beads made of an alginate (MV) with a weight average of 1.2–1.3*106 g/mol. The results were confirmed by the observation that the biocompatibilty of multilayer capsules made of a lower weight average alginate (core and surface LV‐alginate) was significantly impaired compared to multilayer capsules made of an alginate with a higher weight average (core and surface MV‐alginate). It was interesting to note, that not only the weight average of the alginate on the surface of the multilayer capsules defined the extent of tissue fibrosis, but also the weight average of the alginate in the core. It could be noted, that the pro‐fibrotic effect of the LV‐alginate was only partially reversed by an alginate with a higher weight average (MV‐alginate) on the surface of the multilayer capsules. On the basis of the raised data we suggest, that the molecular weight of alginate plays a role in the induction of tissue fibrosis, beside the undisputed role of endotoxin‐and mitogen‐levels (Zekorn et al., [Citation1992]; Zimmermann et al., [Citation1992]).
While the exact physiological and biochemical mechanisms underlying these effects are not fully understood, there is evidence that low molecular weight fractions such as oligosaccharides are involved in this process. It has been reported by Bayer et al. ([Citation1992]) that alginate lyase treatment of mucoid P. aeruginosa improves the efficacy of administered antibiotics and enhances the phagocytosis by host cells (Eftekhar and Speert, [Citation1988]). The latter effect appears a direct result of the a higher yield of alginate oligosaccharides after treatment (Mai et al., [Citation1993]) rather than any specific influence of chemoatractants such as interleukin 8 (Konig et al., [Citation1995]). Furthermore, alginate oligosaccharides possess potent signalling and growth stimulatory effects in plants (Ryan, [Citation1987]) and mammalian cells, such as human keratinocytes and human endothelial cells (Kawada et al., [Citation1997], [Citation1999]). On the basis of the presented data, we assume that oligosaccharides which are non‐bound during the polymerisation process of the alginate bead, are jointly responsible for the microcapsule‐induced inflammatory reaction. Therefore, the reduction or elimination the low molecular weight fractions (< 10− 5) of the alginate, during the purification process, should be aspired to improve the biocompatibility.
Acknowledgments
The authors thank Viola Slotty for her excellent technical assistance. This work was partially supported by the Innovation Foundation, Rheinland‐Pfalz, Germany (FK: 386261/525) and from the Robert Müller Foundation, Mainz.
References
- Bayer A. S., Park S., Ramos M. C., Nast C. C., Eftekhar F., Schiller N. L. Effects of alginase on the natural history and antibiotic therapy of experimental endocarditis caused by mucoid Pseudomonas aeruginosa. Infect. Immun. 1992; 60: 3979–3985
- Berth G. Methodical aspects of characterization of alginate and pectate by light scattering and viscometry coupled with GPC. Carbohydr. Polym. 1992; 19: 1–9
- Eftekhar F., Speert D. P. Alginase treatment of mucoid Pseudomonas aeruginosa enhances phagocytosis by human monocyte‐derived macrophages. Infect. Immun. 1988; 56: 2788–2793
- Hasse C., Klöck G., Schlosser A., Zimmermann U., Rothmund M. Parathyroid allotransplantation without immunosuppression. Lancet 1997; 350: 1296–1297
- Hillgärtner M., Zimmermann H., Mimietz S., Jork A., Thürmer F., Schneider H., Nöth U., Hasse C., Haase A., Fuhr G., Rothmund M., Zimmermann U. Immunoisolation of transplants by entrapment in 19F‐labelled alginate gels: production, biocompatibility, stability, and long‐term monitoring of functional integrity. Mater.wiss. Werkst.tech. 1999; 30: 783–792
- Kawada A., Hiura N., Shiraiwa M., Tajima S., Hiruma M., Hara K., Ishibashi A., Takahara H. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co‐factor for epidermal growth factor in cell culture. FEBS Lett. 1997; 408: 43–46
- Kawada A., Hiura N., Tajima S., Takahara H. Alginate oligosaccharides stimulate VEGF‐mediated growth and migration of human endothelial cells. Arch. Dermatol. Res. 1999; 291: 542–547
- Konig B., Ceska M., Konig W. Effect of Pseudomonas aeruginosa on interleukin‐8 release from human phagocytes. Int. Arch. Allergy Immunol. 1995; 106: 357–365
- Lehr H. A., van der Loos C. M., Teeling P., Gown A. M. Complete chromogen separation and analysis in double immunohistochemical stains using Photoshop‐based image analysis. J. Histochem. Cytochem. 1999; 47: 119–126
- Mach M. A., Schlosser J., Weiland M., Feilen P. J., Ringel M., Hengstler J. G., Weilemann L. S., Beyer J., Kann P., Weber M. M., Schneider S. Cryopreservation of islets of Langerhans: optimization of protocols using rat pancreatic tissue. J. Exp. Clin. Sci. 2003; 2: 6–21
- Mai G. T., Seow W. K., Pier G. B., McCormack J. G., Thong Y. H. Suppression of lymphocyte and neutrophil functions by Pseudomonas aeruginosa mucoid exopolysaccharide (alginate): reversal by physicochemical, alginase, and specific monoclonal antibody treatments. Infect. Immun. 1993; 61: 559–564
- Martinsen A., Skjak‐Braek G., Smidsrod O. Comparison of different methods for determination of molecular weight and molecular weight distribution of alginates. Carbohydr. Polym. 1991; 15: 171–193
- Ryan C. A. Oligosaccharide signalling in plants. Annu. Rev. Cell Biol. 1987; 3: 295–317
- Schneider S., Feilen P. J., Slotty V., Kampfner D., Preuss S., Berger S., Beyer J., Pommersheim R. Multilayer capsules: a promising microencapsulation system for transplantation of pancreatic islets. Biomaterials 2001; 22: 1961–1970
- Schneider S., von Mach M. A., Kraus O., Kann P., Feilen P. J. Intraportal transplantation of allogenic pancreatic islets encapsulated in barium alginate beads in diabetic rats. Artif. Organs 2003, in press
- Vandenbossche G. M., Remon J. P. Influence of the sterilization process on alginate dispersions. J. Pharm. Pharmacol. 1993; 45: 484–486
- Zekorn T., Klöck G., Horcher A., Siebers U., Wohrle M., Kowalski M., Arnold M. W., Federlin K., Bretzel R. G., Zimmermann U. Lymphoid activation by different crude alginates and the effect of purification. Transplant. Proc. 1992; 24: 2952–2953
- Zimmermann U., Klöck G., Federlin K., Hannig K., Kowalski M., Bretzel R. G., Horcher A., Entenmann H., Siebers U., Zekorn T. Production of mitogen‐contamination free alginates with variable ratios of mannuronic acid to guluronic acid by free flow electrophoresis. Electrophoresis 1992; 13: 269–274
- Zimmermann U., Mimietz S., Zimmermann H., Hillgärtner M., Schneider H., Ludwig J., Hasse C., Haase A., Rothmund M., Fuhr G. Hydrogel‐based non‐autologous cell and tissue therapy. Biotechniques 2000; 29: 564–572
- Zimmermann H., Hillgärtner M., Manz B., Feilen P. J., Brunnenmeier F., Leinfelder U., Weber M., Cramer H., Schneider S., Hendrich C., Volke F., Zimmermann U. Fabrication of homogeneously cross‐linked, functional alginate microcapsules validated by NMR‐, CLSM‐ and AFM‐imaging. Biomaterials 2003; 24: 2083–2096
