Abstract
The mechanical properties of biocompatible microparticles including alginate microspheres and alginate–chitosan microcapsules with different wall thickness were determined using a micromanipulation technique. Single microparticles with diameters of 20–60 µm were compressed to a given deformation and held, and compressed to rupture at different speeds. The corresponding force imposed on them was measured simultaneously by a force transducer. Results showed that the force imposed on these particles increased when they were compressed, but relaxed significantly when they were held. For alginate microspheres, the faster the compression speed was, the greater the force being imposed on them at a given deformation. Alginate–chitosan microcapsules showed less force relaxation when they were held, compared with alginate microspheres. The thicker their wall was, the less significant force relaxation the microcapsules exhibited. The mean rupture force of alginate microspheres increased with the compression speed, but this effect in general became less for alginate–chitosan microcapsules, which depended on their wall thickness. However, the deformation at rupture for all three samples was independent of the compression speed. On average, the alginate–chitosan microcapsules were bigger than alginate microspheres and had a greater rupture force.
Introduction
Both alginate and chitosan are natural polysaccharides. Due to their excellent biocompatibility and biodegradability, as well as their ability to form hydrogel or polyelectrolyte complex under very mild conditions, microparticles (spheres or capsules) made from alginate and chitosan have been widely used as carriers for entrapment of drugs (Aslani and Kennedy, Citation[[1996]]; Gonzalez-Rodrigues et al., Citation[[2002]]; Ribeiro et al., Citation[[1999]]), macromolecules (DeGroot and Neufeld, Citation[[2001]]; Kikuchi et al., Citation[[1997]]; Vandenberg et al., Citation[[2001]]), and cells (Read et al., Citation[[1999]]; Strand et al., Citation[[2000]]; Torre et al., Citation[[2000]]). However, microparticles for such applications should have desirable permeability and mechanical properties. The reason for the latter is that they may be exposed to mechanical forces generated at the sites where they need to function, such as compression in cartilage and bone, tension in muscle and tendon, and shear force in blood vessels, and the release of active ingredients from them can also be triggered by mechanical signals (Kuenl et al., Citation[[2001]]). Previous work on microparticles made from alginate and chitosan mainly focused on their preparation methods (Fundueanu et al., Citation[[1998]]; Ponecelet et al., Citation[[1999]]; Seifert and Phillips, Citation[[1997]]) and their potential applications. Very little information is available on their mechanical properties, particularly for those particles smaller than 60 µm in diameter (Bartkowiak and Hunkeler, Citation[[1999]], Citation[[2000]]; Edward-Levy and Levy, Citation[[1999]]; Gaserød et al., Citation[[1998]], Citation[[1999]]; Ouwerx et al., Citation[[1998]]). A few indirect and direct methods have been employed to measure the mechanical properties or strength of microcapsules (Gaserød et al., Citation[[1999]]; Gaumann et al., Citation[[2000]]; Liu et al., Citation[[1996]]; Ohtsubo et al., Citation[[1991]]; Poncelet and Neufeld, Citation[[1989]]), unfortunately none of these methods is capable of determining the mechanical properties, including strength, of single microcapsules smaller than 60 µm in diameter.
Recently, a novel micromanipulation technique has been developed, which has been used to measure the mechanical properties of a wide range of biological and nonbiological microparticles including microcapsules as small as 1 µm in diameter (Sun and Zhang, Citation[[2001]], Citation[[2002]]; Thomas et al., Citation[[2000]]; Zhang et al., Citation[[1999]]). The principle of this technique is to compress single particles between two parallel planes. When the particles are compressed, the force being imposed on them is measured simultaneously. This technique has been used to measure the mechanical strengths of melamine–formaldehyde and urea–formaldehyde microcapsules (Sun and Zhang, Citation[[2001]], Citation[[2002]]; Zhang et al., Citation[[1999]]). It has been found that both melamine–formaldehyde and urea–formaldehyde microcapsules had a wall with very low permeability and their mean bursting strength did not change with the compression speed significantly. However, recent work based on round-robin testing has demonstrated that the rupture force of alginate beads and microcapsules based on alginate, cellulose sulfate, and polymethylene-co-guanidine varied with the compression speed (Grigorescu et al., Citation[[2002]]). Following these observations, the micromanipulation technique has been applied to measure the mechanical properties of biocompatible microparticles with diameters less than 60 µm, including alginate microspheres and alginate–chitosan microcapsules of varying wall thickness. In particular, the effect of compression speed on the rupture force of these microparticles was investigated.
Materials and Methods
Preparation of Alginate Microspheres
Alginate microspheres with diameters smaller than 60 µm were produced via emulsification/internal gelation (Poncelet et al., Citation[[1999]]). Briefly, 20 mL of 2% sodium alginate (BDH Laboratory Supplier, UK), pH 7.5, was mixed with 1 mL suspension of insoluble calcium carbonate, resulting in a suspension of 25 mM calcium salt in the sodium alginate solution. Twenty-one milliliter of this suspension was dispersed in 100 mL soybean oil containing 2% span 80 by stirring at 250 rpm using a six-blade Rushton turbine for 15 minutes. With continuous agitation, 20 mL soybean oil containing 80 mM glacial acetic acid was then added to the emulsion, liberating divalent calcium for gelation of the sodium alginate. After 5 min, 150 mL of 50 mM calcium chloride solution was added with gentle mixing (100 rpm) into the oil-bead suspension. After complete partitioning of beads to the aqueous phase, oil was discarded, and then microspheres were filtered on a sieve and washed with 1% Tween 80 and distilled water twice respectively. Finally, alginate microspheres were stored in distilled water. Apart from sodium alginate, all other chemicals were purchased from Sigma-Aldrich Company Ltd., UK.
Preparation of Alginate–Chitosan Microcapsules
A batch of alginate microspheres was transferred to a 30 mL solution of chitosan pH 5, containing 300 mM of CaCl2. In order to obtain alginate–chitosan microcapsules of varying wall thickness, chitosan concentrations of 0.1 mg/mL and 3 mg/mL were chosen respectively (C3646, Sigma-Aldrich Company Ltd., UK). The alginate microspheres were immersed in each chitosan solution for 30 min, resulting in alginate–chitosan microcapsules, which were then washed and stored in distilled water.
Micromanipulation Technique
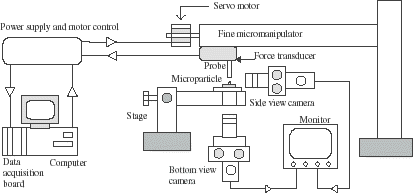
The mechanical properties of microspheres and microcapsules were measured using a micromanipulation technique. The schematic diagram of the micromanipulation rig is shown in . The diameter of the force transducer probe was 125 µm. Details of this technique are described elsewhere (Sun and Zhang, Citation[[2001]]; Zhang et al., Citation[[1999]]). Experiments carried out include single microparticles being compressed to a given deformation and held, and compressed to rupture at different speeds. During the operations, the force imposed on the microparticles was measured simultaneously by a force transducer (Model 403A, Auora Scientific Inc., Canada). The diameters of microparticles were determined from their image under a microscope.
Results and Discussion
Compress and Hold
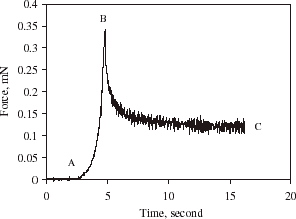
shows the force versus sampling time as a single alginate microsphere was compressed to a certain deformation and then held. Curve AB corresponds to the microsphere being compressed, and BC being held. As can be seen, the force being imposed on the alginate microsphere increased when it was deformed. However, the force relaxed gradually to a certain value when the microsphere was held. It is speculated that alginate microspheres have a matrix structure and high permeability. The relaxation of the force when the alginate microsphere was held might be due to water loss from the microsphere and/or the visco-elastic property of the material. The former may be due to the fact that as the alginate microsphere is compressed its internal pressure is increased, which might result in its internal water being pushing out. The force being imposed on the microsphere when it was compressed, might depend not only on the mechanical rigidity of itself but also on how quickly the water was expelled. Therefore, the effect of the compression speed should be taken into account when such measurements are used to infer the mechanical properties of the material.
Figure 2. Force vs. time for compressing and holding a single alginate microsphere at a compression speed of 8µm/s and final deformation of 66%, diameter = 28 µm.
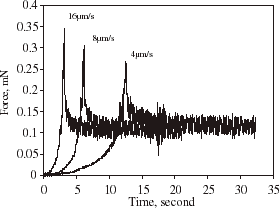
Alginate microspheres were also compressed to a given final deformation at different speeds and then held, and the corresponding curves of force vs. sampling time are shown in . Clearly, the faster the compression speed was, the greater the force imposed on the microsphere at the final deformation. However, the different forces imposed all reached the same level after they were held for a period of time. This might result from that less water was lost at a faster compression speed within the period of compression since the time taken for the microsphere to reach that final deformation was shorter. The force relaxation afterwards was probably mainly due to further loss of water from the microspheres.
Figure 3. Force vs. time for compressing and holding a single alginate microsphere at three different compression speeds.
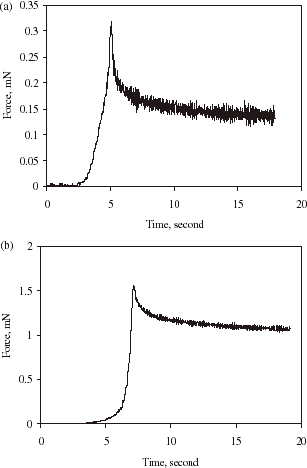
(a) and (b) show typical compression and holding curves of alginate–chitosan microcapsules with relatively thin and thick walls, respectively. As can be seen, the shape of the curves in (a) and (b) are similar to that in , but as the wall thickness was increased, the force relaxation was less significant. This is expected since as the microsphere was coated with a chitosan wall, its permeability should be reduced. The thicker and denser the wall was, the less the permeability. In order to evaluate these phenomena quantitatively, the force relaxation corresponding to curve BC in is fitted by an exponential function as follows.where F is the instantaneous force, F0 the initial force when the microsphere began to be held (i.e., the force at point B), F∞ the force when the time t reaches infinity, and k is a decay constant.
Figure 4. Force vs. time for compressing and holding a single alginate–chitosan microcapsule at a compression speed of 8 µm/s and final deformation of 66%. (a) thin wall and diameter = 33 µm; (b) thick wall and diameter = 53 µm.
The curve BC in is re-plotted in and fitted by Eq. (1) with two parameters F∞/F0 and k using the least squares method. Similarly, from the force relaxation curves in (a) and (b), the corresponding values of F∞/F0 and k have also been determined, and the results are summarized in .
Figure 5. The force relaxation corresponding to curve BC in fitted by an exponential function with two parameters.
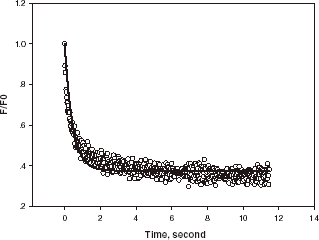
Table 1. F∞/F0 and k of the three samples of microparticles compressed to a deformation of 66%
As can be seen, the value of F∞/F0 increases with the diameter of the microparticle (i.e., the thickness of the chitosan wall), while the decay constant k reduces. This implies that the alginate microsphere (with a wall thickness of zero) had the highest water loss rate which relates to its greatest permeability, largest specific surface area and weakest rigidity, and the alginate–chitosan microcapsules with a thicker and possibly denser (due to being prepared with a higher concentration of chitosan (Gaserød et al., Citation[[1998]])) wall had the lower permeability, smaller specific surface area and stronger rigidity.
Compress Microspheres to Rupture
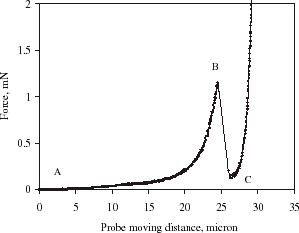
displays a typical curve for compressing a single alginate microsphere to rupture. Point A corresponds to the initial contact between the microsphere and the force transducer probe and curve AB the microcapsule being compressed. The microsphere was ruptured at Point B where there is a significant drop in force. Further movement of the probe resulted in compression of the debris of the ruptured microsphere against the microscope slide, denoted by point C. From the figure, the rupture force and the deformation at rupture (displacement of the microsphere at the rupture divided by its diameter) of each microsphere were determined.
Figure 6. Typical curve showing force vs. probe moving distance as a single microcapsule was compressed to rupture.
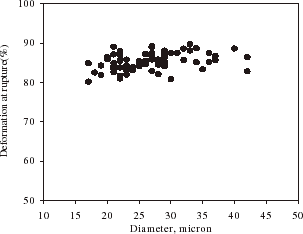
shows the relationship between the rupture force and diameter of single alginate microspheres. On an average, the rupture force increased with the diameter of microspheres. There is a variation of the rupture force for the microspheres of a given size, which might be due to heterogeneity of the matrix structure of the microparticles. gives the deformation at rupture vs. the diameter of single alginate microspheres. It seems the deformation at rupture did not change with the size significantly. Such results are very similar to those for melamine–formaldehyde microcapsules (Sun and Zhang, Citation[[2001]]).
Figure 7. Rupture force vs. diameter of single alginate microspheres at a compression speed of 8 µm/s.
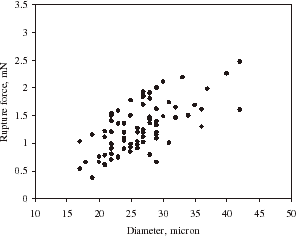
Figure 8. Deformation at rupture vs. diameter of single alginate microspheres at a compression speed of 8 µm/s.
The mean rupture force and deformation at rupture of alginate microspheres measured at different compression speeds are summarized in . As shown, the faster the compression speed was, the greater the mean rupture force. However, the compression speed seemed to have no significant effect on the mean deformation at rupture, with 95% confidence.
Table 2. Mean rupture force of alginate microspheres and deformation at rupture measured at different compression speeds
Alginate–chitosan microcapsules also showed clear rupture under compression. The effect of compression speed on the mean rupture force and deformation at rupture of the microcapsules of varying wall thickness was also determined, and the results are presented in Tables and .
Table 3. Mean rupture force of alginate–chitosan microcapsules with a relatively thin wall and deformation at rupture measured at different compression speeds
Table 4. Mean rupture force of alginate–chitosan microcapsules with a relatively thick wall and deformation at rupture measured at different compression speeds
Compared with alginate microspheres, the alginate–chitosan microcapsules with a relatively thin wall had a greater mean diameter and greater rupture force at a given compression speed. As the speed was increased, the mean rupture force also increased. However, the mean rupture force only increased by 39% when the compression speed varied from 4 to 16 µm/s, compared with 63% for the microspheres shown in . Like the microspheres, the mean deformation at rupture of the alginate–chitosan microcapsules did not change significantly with the compression speed.
When the wall thickness of alginate–chitosan microcapsules was increased, reflected by the increase in the mean diameter, their mean rupture force also increased (also see ). However, both the mean rupture force and the deformation at rupture did not vary with the compression speed significantly. From Tables , , , it can also be seen that the mean deformation at rupture of the microparticles decreased with an increase in the thickness of the coating. This is consistent with the observation that the rigidity of the particles was more dominated by the mechanical property of the chitosan and became stronger (greater value of F∞/F0 and rupture force) as the thickness of the wall was increased.
The difference in the effect of compression speed on the mean rupture force among three samples of microparticles might result from their different rupture mechanisms and their different permeability. For alginate microspheres, their rupture was probably due to the collapse or fracture of their matrix structures (crosslinks). Moreover, they had the highest permeability, and the rapid water loss from them under compression might result in quick reduction of their internal pressure. Whereas for alginate–chitosan microcapsules, their rupture might be caused not only by the collapse or fracture of core matrix structures but also by the yield of the wall material. Their permeability was always lower than that of alginate microspheres as an additional wall was formed, and water loss from them should become slower and their internal pressure rise more rapidly at a given deformation. This explains why the mean rupture force of the alginate–chitosan microcapsules was greater than that of alginate microspheres. Also the mean rupture force of the former was less affected by the compression speed. Furthermore, when the alginate–chitosan microcapsules had a relatively thin wall (), their mechanical strength (reflected by the mean rupture force) might be contributed both by alginate matrix core and the chitosan wall. However, when the alginate–chitosan microcapsules had a thick wall (), their mechanical strength might be dominated by the yielding of the wall material. It is assumed that the difference in diameter between microcapsules and microspheres was equal to twice the thickness of the chitosan wall, the mean wall thickness of alginate–chitosan microcapsules shown in Tables and is estimated to be 1.8 µm and 11 µm, respectively. Since the ratio of wall thickness to radius for the alginate–chitosan microcapsules with the thicker wall is over 40% and the rupture deformation was about 75%, it was quite likely that their walls touched each other before the microcapsules were ruptured (Rehor et al., Citation[[2001]]), which further indicates that the wall material is compressible, and their rupture force was dominated by the yielding of the wall material.
Conclusions
Micromanipulation has been used to determine the mechanical properties of three samples of microparticles including alginate microspheres and alginate microspheres coated with a chitosan wall of varying thickness. It has been found that the force relaxation curve can be fitted well by an exponential function with two parameters, which may be related to both the intrinsic rigidity of the microparticles and the rate of water loss from them under compression. The force imposed on the microspheres at a given deformation or rupture depended on the compression speed significantly. The faster the speed was, the greater the force. The effect of the compression speed on the rupture force of alginate–chitosan microcapsules with a relatively thin wall (mean thickness 1.8 µm) became less, compared with the microspheres. When the microspheres were coated with a relatively thick chitosan wall (mean thickness 11 µm), the compression speed did not have any significant effect on the rupture force. This is believed to result from that the microspheres were most permeable, while the permeability of the microcapsules decreased with an increase in their wall thickness. This work demonstrates the importance of the compression speed on the force required to deform such microparticles to a given deformation or rupture. Future work includes modeling the experimental results in order to determine the intrinsic mechanical property parameters of the materials.
Acknowledgments
Dr Ling Zhao was financially sponsored by The Royal Society KC Wong Fellowship.
References
- Aslani P., Kennedy R. A. Effect of gelation conditions and dissolution media on the release of paracetamol from alginate gel beads. J. Microencapsulation 1996; 13: 601–614, [PUBMED], [INFOTRIEVE], [CSA]
- Bartkowiak A., Hunkeler D. Alginate–oligochitosan microcapsules. I. A mechanic study relating membrane and capsule properties to reaction conditions. Chem. Mater. 1999; 11: 2486–2492, [CROSSREF]
- Bartkowiak A., Hunkeler D. Alginate–oligochitosan microcapsules. II. Control of mechanical resistance and permeability of the membrane. Chem. Mater. 2000; 12: 206–212, [CROSSREF]
- DeGroot A. R., Neufeld R. J. Encapsulation of urease in alginate beads and protection from α-chymotrypsin with chitosan membranes. Enzyme and Microbial Technology 2001; 29: 321–327, [CROSSREF], [CSA]
- Edward-Levy F., Levy M. C. Serum albumin–alginate coated beads: mechanical properties and stability. Biomaterials 1999; 20: 2069–2084, [CROSSREF], [CSA]
- Fundueanu G., Esposito E., Mihai D., Carpov A., Desbrieres J., Rinaudo M., Nastruzzi C. Preparation and characterization of Ca–alginate microspheres by a new emulsification method. Int. J. Pharm. 1998; 170: 11–21, [CROSSREF]
- Gaserød O., Smidsrod O., Skjak-brak G. Microcapsules of alginate–chitosan-I. A quantitative study of the interaction between alginate and chitosan. Biomaterials 1998; 19: 1815–1825, [CROSSREF], [CSA]
- Gaserød O., Sannes A., Skjak-brak G. Microcapsules of alginate–chitosan-II. A study of capsule stability and permeability. Biomaterials 1999; 20: 773–783, [CROSSREF], [CSA]
- Gaumann A., Laudes M., Jacob B., Pommersheim R., Laue C., Vogt W., Schrezenmeir J. Effect of media composition on long-term in vitro stability of barium alginate and polyacrylic acid multilayer microcapsules. Biomaterials 2000; 21: 1911–1917, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Gonzalez-Rodriguez M. L., Holgado M. A., Sanchez-Lafuente C., Rabasco A. M., Fini A. Alginate/chitosan particulate systems for sodium diclofenac release. Int. J. Pharm. 2002; 132: 225–234, [CROSSREF]
- Grigorescu G., Rosinski S., Lewinska D., Ritzén L. G., Viernstein H., Teunou E., Poncelet D., Zhang Z., Fan X., Serp D., Marison I., Hunkeler D. Characterization of microcapsules: recommended methods based on round-robin testing. J. Microencapsulation 2002; 19: 641–649, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kikuchi A., Kawabuchi M., Sugihara M., Sakurai Y., Okano T. Pulsed dextran release from calcium–alginate gel beads. J. Control. Release 1997; 47: 21–29, [CROSSREF]
- Kuen Y. L., Martin C. P., David J. M. Controlled drug delivery from polymers by mechanical signals. Advanced Materials 2001; 13: 837–839, [CROSSREF]
- Liu K. K., Williams D. R., Briscoe B. J. Compressive deformation of a single microcapsule. Physical Review 1996; 54: 6673–6680, [PUBMED], [INFOTRIEVE]
- Ohtsubo T., Takeda H., Tsuda S., Kagoshima M., Tsuji K. A study of the physical strength of fenitrothion microcapsules. Polymer 1991; 32: 2395–2399, [CROSSREF]
- Ouwerx C., Veilings N., Mestdaghand M. M., Axelos M.A.V. Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Networks 1998; 6: 393–408, [CROSSREF]
- Poncelet D., Neufeld R. T. Shear breakage of nylon membrane microcapsules in a turbine reactor. Biotechnol. Bioengng. 1989; 33: 95–103
- Ponecelet D., Babak V., Dulieu C., Picot A. A physico-chemical approach to production of alginate beads by emulsification-internal ionotropic gelation. Colloids and Surfaces 1999; 155: 171–176, [CROSSREF]
- Read T., Stensvaag V., Vindenes H., Ulvestad E., Bjerkvig R., Thorsen F. Cells encapsulation in alginate: a potential system for delivery of recombinant proteins to malignant brain tumours. Int. J. Neurosicence 1999; 7: 653–663, [CROSSREF]
- Rehor A., Canaple L., Zhang Z., Hunkeler D. The compression deformation of multicomponent microcapsules: Influence of size, membrane thickness and compression speed. J. Biomater. Sci. Polymer Edn. 2001; 12: 157–170, [CROSSREF]
- Ribeiro A. J., Neufeld R. J., Arnaud R. J., Chaumeil J. C. Microencapsulation of lipophilic drugs in chitosan-coated alginate microspheres. Int. J. Pharm. 1999; 187: 115–123, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Seifert D. B., Phillips J. A. Production of small, monodispersed alginate beads for cell immobilization. Biotechnol. Prog. 1997; 13: 562–568, [CROSSREF], [CSA]
- Strand B. L., Morch Y. A., Skjak-braek G. S. Alginate as immobilization matrix for cells. Minerva Biotec. 2000; 12: 223–233, [CSA]
- Sun G, Zhang Z. Mechanical properties of melamine–formaldehyde microcapsules. J. Microencapsulation 2001; 18: 593–602, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sun G., Zhang Z. Mechanical strength of microcapsules made of different wall materials. International Journal of Pharmacy 2002; 242: 307–311, [CROSSREF]
- Thomas C. R., Zhang Z., Cowen C. Micromanipulation measurements of biological materials. Biotechnology Techniques 2000; 22: 531–537
- Torre M. L., Maggi L., Vigo D., Galli A., Bornaghi V., Maffeo G., Conte U. Controlled release of swine semen encapsulated in calcium alginate beads. Biomaterials 2000; 21: 1493–1498, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Vandenberg G. W., Drolet C., Scott S. L., Noue J. de la. Factors affecting protein release from alginate–chitosan coacervate microcapsules during production and gastric/intestinal simulation. J. Controlled Release 2001; 77: 297–307, [CROSSREF], [CSA]
- Zhang Z., Saunders R., Thomas C. R. Mechanical strength of single microcapsules determined by a novel micromanipulation technique. J. Microencapsulation 1999; 16: 117–124, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]