Abstract
The kinetics of both malolactic fermentation in Chardonnay wine by encapsulating Lactobacillus casei cells in pectate gel and lyophilized Oenococcus oeni culture has been carried out. The influence of acidity, sulfur dioxide content, and organic acid content on the malolactic activity of the bacteria has been controlled. Encapsulated bacteria degraded 30% of malic acid in white wine, deacidifying it from pH 3.15 to 3.40, whereas the lyophilized culture degraded 48% of malic acid, deacidifying from pH 3.15 to 3.60. The degree of conversion of malic acid in wine by the encapsulated cells was twice as high as that obtained by the free Lactobacillus casei cells. The operational stability of calcium pectate gel capsules was 6 months. It has been proved that the encapsulated biocatalyst increases the rate of fermentation, and induces the fermentation to take place at high ethanol concentrations. The proposed encapsulated biocatalyst is an attractive material for industrial applications in continuous winemaking processes.
Introduction
One of the most difficult steps to control in winemaking is malolactic fermentation (MLF), which normally occurs after completion of the alcoholic fermentation. It is conducted by lactic acid bacteria (LAB) Oenococcus oeni, Lactobacillus casei, or Pediococcus (Kunkee, Citation[[1991]]; Lafon-Lafourcade, Citation[[1975]]). LAB deacidify the wine by converting malic acid to lactic acid resulting in a wine with improved organoleptic quality (Nielsen and Richelieu, Citation[[1999]]).
The use of immobilized cells in different matrices for the control of MLF in wine is now confirmed by substantial research, but no industrial installation is yet operating to our knowledge. Entrapment of cells represents one possible solution for immobilization of LAB. The main supports/matrixes used consist of polyacrylamide gel, calcium alginate, κ-carrageenan, and cellulose sponge (Maicas, Citation[[2001]]).
Commercial encapsulation technologies in the food industry have existed for over a decade and show an alternative solution for biocatalyst preparation in winemaking. Under the terms of the 1995 EC Directive, a wide range of additives in foodstuffs is permitted (Hopkinson, Citation[[2001]]). Alginate has been used as a popular material for encapsulation (Poncelet and Markvicheva, Citation[[2001]]). Two strains of yeast immobilized in a double-layer alginate gel were used for the control of simultaneous deacidification and alcohol fermentation during production of white wine (Yajima and Yokotsuka, Citation[[2001]]; Yokotsuka et al., Citation[[1993]], Citation[[1997]]).
On the other hand, lyophilization is considered to be a valuable method for preservation of foods and to a greater extent for microorganisms. Bakatorou et al. demonstrated that the lyophilized immobilized cells (yeast) on gluten pellets may give a possibility for the development of a marketable process for the preservation of immobilized cells in order to supply wineries with freeze-dried ready-to-be-used cells (Bakatorou et al., Citation[[2001]]).
Lyophilized industrial cultures of O. oeni for induction of MLF in wine have been available for some years. Nielsen et al. (Citation[[1996]]) demonstrated for the first time that a freeze-dried preparation of a selected L. oenos can survive 100% in direct inoculation into wine and induce MLF quickly under common vinification conditions in the laboratory. The immediate survival and the lag phase of the inoculated bacteria are of critical importance because these two factors determine the total duration of MLF.
The aim of this paper is a comparative study of MLF in Bulgarian Chardonnay wine by free and encapsulated L. casei cells as well as lyophilized cultures of Oenococcus oeni. We selected calcium pectate gel as the encapsulation material to accomplish MLF in wine. The application of pectate gel in winemaking holds good prospects due to its good stability at low pH values. Also, pectin is among the polysaccharides permitted by the European legislation as a potential encapsulation “carrier/support” for food additive systems.
Materials and Methods
The L. casei (NBIMCC 1013) strain, cultivation media and growth conditions have been previously described (Kosseva et al., Citation[[1995]]). The L. casei cells were encapsulated into calcium pectate gel beads. The commercial citrus pectin (BDH) solution (5.5% w/v in water) was neutralized with concentrated ammonia to pH 7–7.5. Harvested cells (0.17 g wet wt) suspension was diluted with sterile distilled water and added to the ammonium pectate solution to a final concentration of 5% (v/v). The mixture of cells and ammonium pectate solution obtained was dripped by means of a syringe into a stirred precipitation bath (0.2 M CaCl2, 0.1 L). The beads formed were incubated in the precipitation bath overnight at 4°C (Kurillova et al., Citation[[1992]]). Afterwards the beads were treated with 0.1 M aluminium nitrate solution for 5 min in order to form a hard surface layer and improve the mechanical strength of the particles. Finally, beads were washes with isotonic solution (Kosseva et al., Citation[[1991]]).
Lyophilized mixed culture of O. oeni (Erbsloh Geisenheim, Germany) (16.54 g) was suspended in sterile water (0.10 L), using a shaking machine for 1 h at 17°C, according to the supplier's instructions. Then rehydrated culture (0.01 L) was inoculated to white wine (0.10 L) for conducting MLF.
The Chardonnay wine samples were kindly supplied by Bulgarian Wine Company. Batch experiments were conducted with wine samples I, II, and III taken after alcoholic fermentation and filtration, as follows: I without SO2; II contained free SO2 (19 mg/L) and total SO2 (88 mg/L); III contained free SO2 (25 mg/L) and total SO2 (80 mg/L).
The kinetics of the MLF were studied in shake flasks at 25 and 20°C using a rotary shaker (New Brunswick Scientific, USA) at 160 rpm. Each volume of the white wine samples (0.10 and 0.17 L) were treated with the encapsulated L. casei cells (0.05 L) or inoculated with free cells (10% inoculum equal to 0.08–0.10 g dry weight of biomass). The ethanol content was 12% (v/v) in samples I and II, and 13.5 % (v/v) in sample III.
Analysis of the organic acids and ethanol in white wine samples was accomplished by HPLC, using a Perkin Elmer chromatograph, series 10, supplied with refractive-index and ultraviolet detectors. A Bio-Rad Aminex HPX-87H column (300 × 7.8 mm) packed with a sulfonated divinylbenzene-styrene copolymer was used for the separation of compounds. The mobile phase, 0.005 M H2SO4, was fed at a flow rate of 0.5 mL/min. The run time of a sample was 30 min.
Results and Discussion
In our previous paper (Kosseva et al., Citation[[1998]]), we described that L. casei bacteria metabolize sugars, glycerol, and organic acids contained in white Chardonnay wines. Those bacteria transform hexoses to lactic acid, using the homofermentative pathway. Also, the metabolism involves the conversion of glycerol and malic acid to lactic, succinic and acetic acid.
Kinetic curves of organic acid evaluation in wine sample I with free L. casei cells at 25°C () show that during the first hour a decrease in the concentration of malic acid was observed whereas the concentrations of lactic, succinic, and acetic acids increased. Afterwards a slight decrease in the concentrations of all acids was observed; further fluctuations of the above concentrations were observed during the next 20–24 h.
Figure 1. Kinetic curves of organic acid evaluation in a sample of Bulgarian Chardonnay wine (sample I) with free cells of L. casei at 25°C.
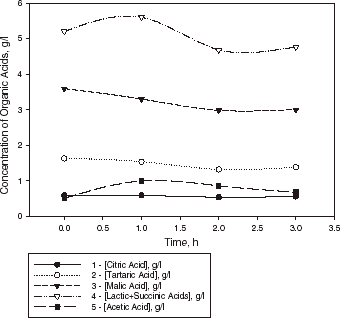
Kinetic curves of organic acid evaluation in wine sample I with L. casei cells encapsulated in calcium pectate gel at 25°C are shown in . This illustrates that one of the benefits of the application of encapsulated cells is the higher degree of conversion of malic acid reached by encapsulated cells (30%), whereas conversion by free cells is approximately 15%. Also, change in acidity of the above wine samples from initial pH = 3.2 to final pH = 3.4–3.5 was observed after 1 h of fermentation. Fluctuation of the concentrations of acids in wine sample II with encapsulated cells during the next 22 h of the fermentation is shown in . The decrease of malic acid observed in this sample is 23%. The data obtained showed that the ability of L. casei to metabolize malic acid in wine can fluctuate with time not only for free cells in suspended culture but also for the immobilized cell system (Kosseva et al., Citation[[1998]]; Spettoli et al., Citation[[1987]]). The results on the analysis of the percentage of the malic acid decrease refer to the fermentation after 20–24 h when the activity of cells had reached a plateau.
Figure 2. Kinetic curves of organic acid evaluation in a sample of Bulgarian Chardonnay wine (sample I) with L. casei cells encapsulated in calcium pectate gel (1 g dry weight of biomass) at 25°C.
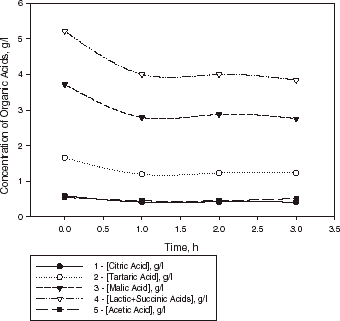
Figure 3. Kinetic curves of organic acid evaluation in a sample of Bulgarian Chardonnay wine (sample II) with L. casei cells encapsulated in calcium pectate gel (1 g dry weight of biomass) at 25°C.
One of the possible explanations of lactic acid evolution during our study is the presence of Acetobacter sp., which may decrease a concentration of lactic acid due to it oxidation to acetic acid. These unwanted bacteria may occur due to the nonaseptic conditions during our experiments with encapsulated cells at 20 and 25°C. Partial retention of organic acids to the encapsulated matrix might occur as well.
The degradation of malic acid observed after spontaneous MLF during one year of storage at 20°C is 5.8% and the final pH value is 3.2 in this case.
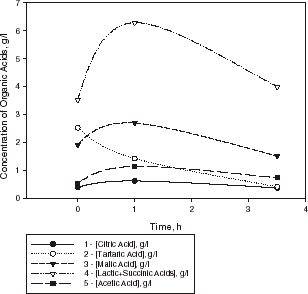
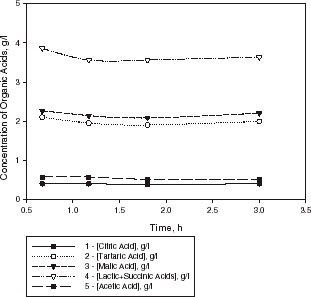
The course of MLF carried out by lyophilized O. oeni culture in sample III is shown in . During the first hour, a decrease in the concentration of tartaric acid was observed, while concentrations of lactic, malic, and acetic acids increased. After 1 h, the concentration of tartaric acid further decreased, while the concentrations of lactic and malic acids began to decrease too. An optimum coefficient of acid harmony, defined as the ratio of the concentrations of tartaric to malic acid (about 2.0) was obtained in 4.5 h. At the same time the degree of conversion of malic acid was about 48% in wine sample III (which contained sulfur dioxide). The acidity of this wine sample changed from pH 3.15 to 3.6.
Figure 4. Kinetic curves of organic acid evaluation in a sample of Bulgarian Chardonnay wine (sample III) with lyophilized culture of O. oeni at 20°C.
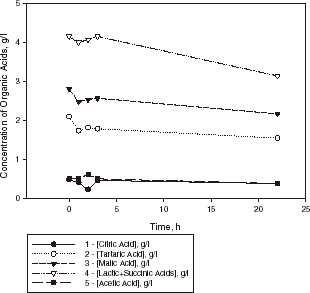
For the sake of comparison, kinetic curves of the organic acids evaluation in sample III with cells encapsulated in pectate gel at 20°C are shown in . In this case, the decrease in malic acid was approximately 5% and the deacidification observed (from pH 3.15 to 3.40) was due to tartaric acid degradation (about 20%). In sample III the coefficient of acid harmony was about unity. In this case, the reason for the lower malolactic activity of the encapsulated L. casei is the temperature (20°C), which is the main factor affecting degradation of organic acids. The optimum temperature of the above strain is 36°C.
Figure 5. Kinetic curves of organic acid evaluation in a sample of Bulgarian Chardonnay wine (sample III) with L. casei cells encapsulated in calcium pectate gel (1 g dry weight of biomass) at 20°C.
Common to the industrial starter culture of O. oeni is the fact that loss of viability is very high if the cultures are inoculated directly into wine. Starter cultures need one or more steps of reactivation and adaptation to wine before use in order to enhance the survival of the bacteria when inoculated into the wine (Nielsen et al., Citation[[1996]]). These steps, which are very time and labor consuming, have limited the practical application of starter cultures. That is why the inoculation with lyophilized commercial strains for induction of MLF or application of technology of encapsulated cells in winemaking is still controversial. Industrial benefits of encapsulation include enhanced fermentation productivity, cell stability and lower costs of down stream processing (Groboillot et al., Citation[[1994]]). Cell encapsulation can be used for the control of MLF and offers the following advantages: continuous operation, greater tolerance to wines, better control over the timing and extent of acidification. Finally, the growth of the bacteria and the time required to complete MLF depend on how the bacteria are affected by the inhibitory components of the wine such as pH, ethanol, and sulfur dioxide. Encapsulation of LAB is an advantage preventing those inhibitory effects of the medium. Encapsulation of the viable bacteria after production on an optimized growth medium helps avoiding the difficult and uncertain phase of growth. Operational stability of the cells encapsulated in the calcium pectate gel is approximately 6 months after use in eight batch fermentations. The proposed encapsulated biocatalyst is therefore an attractive material for industrial application in continuous winemaking.
Conclusions
Industrial production of grape wine in continuous processes depends upon the food-grade purity of the support, taste, and aroma of the wine and cost-effective encapsulation procedure. Encapsulation in pectate gel is a safe, mild, cheap, and versatile technique, which may be used for the preparation of biocatalyst for MLF in wine. Encapsulated bacteria of L. casei degraded 30% of malic acid in white wine, deacidifying it from pH 3.15 to 3.40, whereas the lyophilized culture of O. oeni degraded 48% of malic acid, deacidifying from pH 3.15 to 3.60. The degree of conversion of malic acid in wine was twice as high as that obtained by free cells. The operational stability of calcium pectate gel capsules was 6 months. Also, the presence of free and total sulfur dioxide did not affect malolactic activity of encapsulated cells. It has been proved that the encapsulated biocatalyst increases the rate of fermentation, and makes the fermentation take place even at high ethanol concentrations (12–13% (v/v)).
Acknowledgment
The authors acknowledge the financial support of this work by the British Council in Sofia, under the Academic Link Sof/1002/22.
References
- Bakatorou A., Soupioni M. J., Koutinas A. A. Freeze-dried Saccharomyces cerevisiae immobilized on gluten pellets for glucose fermentation. Process Biochemistry 2001; 36: 549–557
- Groboillot A., Boadi D. K., Poncelet D., Neufeld R. J. Immobilisation of cells for application in food industry. Crit. Rev. Biotechnol. 1994; 14: 75–107, [PUBMED], [INFOTRIEVE], [CSA]
- Hopkinson L. M. Microencapsulation of Food Ingredients, P. Vilstrup. Leatherhead Publishing, SurreyEngland 2001; 76–78
- Kosseva M., Beschkov V., Kennedy J. F., Lloyd L. L. Malolactic fermentation in Chardonnay wine by immobilised Lactobacillus casei cells. Process Biochemistry 1998; 33: 793–797, [CROSSREF]
- Kosseva M. R., Beschkov V. N., Pilafova E. I. Lactic acid production from lactose by immobilised Lactobacillus casei Cells. Bulgarian Chemical Communications 1995; 28: 690–703
- Kosseva M., Beschkov V., Popov R. Biotransformation of D-sorbitol to L-sorbose by immobilized cells of Gluconobacter suboxydans in a bubble column. J. Biotechnol. 1991; 19: 301–308, [CROSSREF]
- Kunkee R. E. Some roles of malic acid in the malolactic fermentation in winemaking. FEMS Microbiology Reviews 1991; 88: 55–72, [CROSSREF], [CSA]
- Kurillova L., Gemeiner P., Ilavsky M., Stefuca V., Polakovic M., Welwardova A., Toth D. Calcium pectate gel beads for cell entrapment. Biotechnol. Appl. Biochem. 1992; 16: 236–251
- Lafon-Lafourcade S. Lactic Acid Bacteria in Beverages and Food, J. G. Carr, C. Y. Cutting, G. C. Whiting. Academic Press, London 1975; 43–53
- Maicas S. The use of alternative technologies to develop malolactic fermentation in wine. Appl. Microbiol. Biotechnol 2001; 56: 35–39, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Nielsen J. C., Prahl C., Lonvaud-Funel A. Malolactic fermentation in wine by direct inoculation with freeze-dried Leuconostoc oenos cultures. Amer. J. Enol. Vitic. 1996; 47: 42
- Nielsen J. C., Richelieu M. Control of flavour development in wine during and after malolactic fermentation by Oenococcus oeni. Appl. Envir. Microbiol. 1999; 65: 740–745, [CSA]
- Poncelet D., Markvicheva E. Microencapsulation of Food Ingredients, P. Vilstrup. Leatherhead Publishing, SurreyEngland 2001; 215–223
- Spettoli P., Nuti M. P., Crapisis A., Zamorani A. Technological improvement of malolactic fermentation in wine by immobilized microbial cells in a continuous flow reactor. Enzyme Engineering 1987; 8: 386–389, [CSA]
- Yajima M., Yokotsuka K. Volatile compound formation in white wines fermented using immobilized and free yeast. Am. J. Enol. Vitic. 2001; 52(3)210–218
- Yokotsuka K., Otaki A., Naitoh A., Tanaka H. Controlled simultaneous deacidification and alcohol fermentation of a high-acid grape must using 2 immobilized yeasts Schizosaccharomyces pombe and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1993; 44(4)371–377
- Yokotsuka K., Yajima M., Matsudo T. Production of bottle-fermented sparkling wine using yeast immobilized in double-layer gel beads or strands. Am. J. Enol. Vitic. 1997; 48(4)471–481