Abstract
Enzyme encapsulation into liposomes is a promising technique to stabilize and prevent them from denaturation and proteolysis. We demonstrate this using acetylcholinesterase which is the main target for pesticides. In order to achieve a reasonable encapsulation yield, we analyzed the parameters involved in each step of various encapsulation procedures. The only encapsulation method which did not denature the protein was the lipid film hydration technique, however the encapsulation efficiency was usually low. The efficiency could be increased up to more than 40% by induction of a specific interaction between the enzyme and the lipid surface. Once encapsulated, the enzyme encountered another problem: the permeability barrier of the lipid membrane drastically diminished the activity of the enzyme entrapped in the liposome by reducing the entrance rate of the substrate molecules and then reducing the substrate concentration inside the liposome. To solve this problem, we controlled the permeability of the liposome wall by reconstituting a porin from Escherichia coli. We succeeded to recover the full functionality of the enzyme, while retaining the protection against denaturation and proteolytic enzymes.
Introduction
Genomics and recent developments in molecular biology have created a large variety of proteins, and notably enzymes that are useful for biotechnological applications regarding biosensors, protein chips, and biocatalysts. However, most proteins or enzymes are fragile, and small conformational changes may reduce their activity. Therefore, their stabilization is required. One possibility is to encapsulate the protein into nanometer sized vesicles to protect it from denaturation by proteolysis and dilution effects. However, the existing encapsulation protocols have been optimized to achieve high encapsulation of chemically stable molecules and are not suitable for the preservation of enzyme functionality (Lichtenberg et al., Citation[[1998]]; Moscho et al., Citation[[1996]]). We found that the method which denatures less protein was the lipid film hydration technique, but encapsulation efficiency was low (Anselem et al., Citation[[1993]]; Kirby and Gregoriadis, Citation[[1984]]). One of our aims was to improve the film hydration method for loading liposomes with proteins, while preserving their functionality. We chose to encapsulate Drosophila melanogaster acetylcholinesterase (AChE) as a reporter enzyme because of the availability of an easy and accurate functional assay. Moreover, this type of enzyme is highly interesting as a biosensor to detect insecticide residues (Villatte et al., Citation[[1998]]). Here we demonstrate that 40% of the initial active enzyme can be encapsulated in its fully functional state.
On the other hand, the liposome encapsulated enzyme has an apparent reduced activity. The lipid membrane reduces the permeation of substrate leading to a reduced concentration inside the liposome. To solve this problem, we control the permeability of the liposome wall by reconstituting a porin from Escherichia coli. We succeeded to recover the full function of the enzyme, while retaining protection against denaturation and proteolytic enzymes (Nasseau et al., Citation[[2001]]).
Materials and Methods
Lipids
1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphoserine (POPS), 1,2-Dioleoyl-sn-Glycero-3-{[N(5-Amino-1-Carboxypentyl)iminodiAcetic Acid]Succinyl} (Nickel salt) (DOGS-NTA-Ni), were from Avanti Polar Lipids, Inc. (Alabaster, AL) and eggPC extract (98% pure) was from Lipoïd (Ludwigshafen, Germany).
Proteins
Pronase was purchased from Sigma (St. Louis, MO). Recombinant acetylcholinesterase was produced in baculovirus infected cells and purified by affinity chromatography (Estrada-Mondaca and Fournier, Citation[[1998]]). Drosophila AChE is a dimer which is linked to the membrane via a glycolipid anchor. This form can be converted to a hydrophilic dimer by expression of a truncated cDNA (Mutero and Fournier, Citation[[1992]]). This hydrophilic form was used. Furthermore, each monomer of the dimer was tagged with three histidines by site directed mutagenesis. The porin OmpF expressed and purified from E. coli is a trimer with each monomer of about 40 kDa and allows the passive diffusion of small solutes below 400 Da as MW (Van Gelder et al., Citation[[2000]]).
Preparation of Liposomes
Liposomes were prepared using the film hydration method. Under standard conditions, 5 mg of lipids in chloroform solution were dried at the bottom of an 8 mL tube for 15 min under nitrogen stream and desiccated for 6 h under vacuum. Multilamellar vesicles were obtained by hydration of the film with 0.1 mL of 25 mM MOPS buffer pH 8.5, containing 0.3 nM of enzyme. The tube was vortexed until the lipid film peeled off from the tube surface. To break the multilamellar vesicles into monolamellar, ten cycles of freezing (liquid nitrogen) and thawing (30°C water bath) were applied. The sample was then diluted to 1 mL in 25 mM MOPS buffer pH 8.5. Size of liposome was homogenized by extrusion by passing the sample 10-fold through a 200 nm pore polycarbonate filter. Modifications of this method are indicated for each experiment.
Quantification of the Encapsulation Efficiency
AChE activity is measured using the Ellman reaction (Ellman et al., Citation[[1961]]). AChE catalyzes the hydrolysis of acetylthiocholine, producing acetate and thiocholine. Thiocholine is then able to react with di-thionitrobenzoic acid, producing a thiocholine coupled to a thio-nitro-benzoic acid by a disulfide bond and a thio-nitro-benzoate. This molecule is detectable by O.D. measurements at 412 nm with an ε = 13,600 M−1cm−1. AChE activity is equal to the absorbance variation plotted vs. time.
To determine the fraction of encapsulated AChE, the solution was incubated in a solution of the proteolytic enzyme pronase. Pronase does not pass through the liposomal bilayer and therefore the encapsulated AChE is protected from enzymatic digestion. Free enzyme and enzyme bound to the external surface of the liposome are inactivated according to first order kinetics. The proportion of encapsulated enzyme was estimated by recording the remaining activity with 1 mM acetylthiocholine in the presence of 0.1% Triton X-100. Mixed lipid–detergent micelles were found to be reversible inhibitors of AchE. Minor inhibition kinetics of AChE by POPS and eggPC were recorded and the results shown in this paper take those inhibitions into account. The full activity of AChE was considered after correction by its rate of inhibition in the conditions of each experiment.
Results
Effect of Lipid Concentration
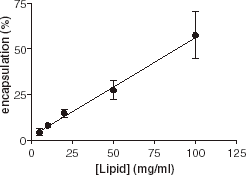
In the first series of experiments, we investigated the influence of lipid concentration on encapsulation. Various amounts of eggPC (from 0.5 to 10 mg in 0.1 to 1 mL buffer) were used and the encapsulation efficiency of AChE was recorded. clearly shows a linear relationship between the lipid concentration and the encapsulation efficiency. Increase of the lipid concentration leads to the formation of more vesicles.
Effect of Freeze–Thaw Cycles
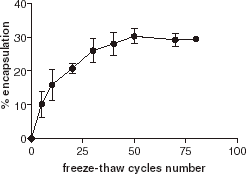
Hydration of lipid films results in inhomogeneous multilamellar vesicles. The application of a series of freeze–thaw cycles breaks the multilamellar vesicles into unilamellar vesicles. As a freeze–thaw cycle usually causes some denaturation of proteins, we investigated the effect of the number of freeze–thaw cycles on encapsulation. As shown in , we observed an increase in encapsulation efficiency according to an increase in the number of freeze–thaw cycles, without significant denaturation of the enzyme. This suggests that encapsulation mainly occurs during the freeze–thaw step. After 50 freeze–thaw cycles, the encapsulation efficiency reaches a plateau that is the maximum for encapsulation.
Influence of the Extrusion Filter Composition
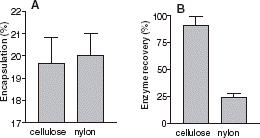
The removal of nonencapsulated enzyme can be done by incubating the liposome solution with pronase, using affinity column chromatography or using gel filtration. However these techniques are time consuming. Nylon is known to be a very efficient adsorption support for proteins in many biological techniques (Western blot, …). shows that a nylon filter could be a rapid method to remove nonencapsulated enzyme. Compared to cellulose acetate, usually used for extrusion, a nylon filter does not affect the encapsulation efficiency. We also compared the final total (encapsulated and free enzyme) activity after extrusion, with the initial total activity. This gives the enzyme recovery. This allows an estimate to be made on the amount of enzyme lost, either adsorbed inside the filter or denatured. Compared to cellulose acetate, enzyme recovery was weak using a nylon filter. The nylon filter allows removal of nonencapsulated enzymes without any detrimental effect on encapsulation.
Figure 3. (A) Effect of filter composition on AChE encapsulation percentage: encapsulated enzymatic activity/total activity. (B) Enzyme recovery percentage: Total enzymatic activity after extrusion/total enzymatic activity. The filter used: Cellulose Acetate (first column) and Nylon (second column).
Activity of the Encapsulated Enzyme
Acetylcholinesterase hydrolyzes its substrate within a wide range of concentrations from 1 μM to 100 mM. In the kinetics (closed circle) shows an activation at low substrate concentration and an inhibition at high substrate concentration, as previously described. In a control measurement, we incorporated AChE into liposomes without porin. The enzyme retained some activity and gave a similar activity-substrate profile as the free enzyme (open circle). This activity was due to encapsulated enzyme and not to extraliposomal enzyme, since it was resistant to protease. This suggests that acetylthiocholine and the product of the reaction, thiocholine, do cross the lipid bilayer but the permeation through the lipid bilayer became the rate-limiting step of the encapsulated enzyme. In the following step we encapsulated acetylcholinesterase into liposomes with reconstituted general diffusion porin OmpF to control the translocation of the substrate (MW 162 Da) and its products. No difference in the specific activity of the encapsulated enzyme (open squares) is visible, demonstrating clearly the absence of the membrane as a permeation barrier.
Protease Protection
Encapsulation of enzymes into liposomes prevents the degradation of proteins by proteases. This protection originates from the membrane barrier since solubilization of liposomes by 0.1% Triton X-100 restored the proteolysis susceptibility. OmpF itself is known to be highly resistant against proteases.
Discussion
We showed that enzyme encapsulation increases with an increase in lipid concentration. Freeze–thaw cycles are shown to be the main step to increase enzyme entrapment. This augmentation is due to the transformation of multilamellar vesicles into monolamellar ones, that is usually achieved after a few cycles. We hypothesized that the freezing of the inner aqueous volume of the liposomes leads to a volume increase that disrupts their membranes. Then during the thawing the AChE diffuses through the disruption from the external solution to the liposome inner volume, until the concentrations of enzyme equilibrate on each side of the membranes. The use of nylon filters for extrusion appears to be a rapid method to remove nonencapsulated enzyme.
Furthermore, functionalized liposomes with reconstituted porins can be used to protect the encapsulated enzymes from protease degradation and denaturation while retaining their activity.
According to these results, we derived the following protocol to achieve up to 40% encapsulation of 0.3 nmol of protein in liposomes permeabilized with OmpF:
Prepare the lipid film
Dry 5 mg of eggPC in chloroform solution at the bottom of an 8 mL tube using a nitrogen stream and desiccate it for 6 h under vacuum.
Add onto the lipid film 10 µL of 25 mM MOPS pH 8.5, 10 mM EDTA, 1% octyl-POE (n-octyloligoOxyEthylene detergent) containing 1 mg/mL OmpF.
Solubilize the lipid film in the OmpF solution using a vortex. If necessary, add some more OmpF solution buffer if the lipid film solubilization is difficult.
Dry this lipid suspension again as described in step 1.Prepare the multilamellar liposomes
Add onto the film 0.1 mL of 25 mM MOPS pH 8.5 containing 0.3 nmol AChE.
Vortex until the lipid film peels off from the tube surface and solubilizes.Break the multilamellar liposomes into monolamellar liposomes
Apply 10 freezing (liquid nitrogen) and thawing (30°C waterbath) cycles to the multilamellar liposome solution.Calibrate the liposomes
Add 0.9 mL of 25 mM MOPS pH 8.5 to the monolamellar liposome solution.
Extrude this solution, passing it 10 times through a 200 nm pore polycarbonate filter.Remove the nonencapsulated enzyme
Add 50 μL of a 50 mg/mL protease solution to the liposome solution, and incubate for 3 h. Or perform a gel filtration and recover the turbid fractions containing the liposomes. Or use nylon filters to achieve step 6 (except if proteins such as OmpF are incorporated in the liposome membrane composition).
References
- Anselem S., Gabizon A., Barenholz Y., Gregoriadis G. Liposome Technology. CRC Press, Boca Raton, FL 1993
- Ellman G. L., Courtney K. D., Andres K. D., Featherstone R. M. A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem. Pharmac. 1961; 7: 88–95
- Estrada-Mondaca S., Fournier D. Stabilization of recombinant Drosophila acetylcholinesterase. Prot. Expr. Purif. 1998; 12: 166–172, [CROSSREF], [CSA]
- Kirby C., Gregoriadis G. Dehydratation–rehydratation vesicles: a simple method for high yield drug entrapment in liposomes. Biotechnology 1984; 2: 979–984, [CROSSREF]
- Lichtenberg D., Barenholtz Y. Liposomes: preparation, characterization and preservation. Methods Biochem. Anal. 1998; 33: 337–462
- Moscho A., Orwar O., Chiu D. T., Modi B. P., Zaren R. N. Rapid preparation of giant unilamellar vesicles. Proc. Natl. Acad. Sci. USA 1996; 93: 11443–11447, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mutero A., Fournier D. Post-translational modifications of Drosophila acetylcholinesterase. In vitro mutagenesis and expression in Xenopus oocytes. J. Biol. Chem. 1992; 267: 1695–1700, [PUBMED], [INFOTRIEVE]
- Nasseau M., Boublik Y., Meir W., Winterhalter M., Fournier D. Substrate-permeable encapsulation of enzymes maintains effective activity, stabilizes against denaturation, and protects against proteolytic degradation. Biotechnol. Bioeng. 2001; 75: 615–618, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Van Gelder P., Dumas F., Winterhalter M. Understanding the function of bacterial outer membrane channels by reconstituting into black lipid membranes. Biophys. Chem. 2000; 85: 153–167, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Villatte F., Marcel V., Estrada-Mondaca S., Fournier D. Engineering sensitive acetylcholinesterase for detection of organophosphate and carbamate insecticides. Biosens. Bioelectr. 1998; 13: 157–164, [CROSSREF], [CSA]
![Figure 4. Specific activity (v/[Et]) of acetylcholinesterase as a function of acetylthiocholine concentration outside the liposome. Three enzyme formulations were tested, free AChE in absence of liposome, encapsulated AChE in liposomes with or without the porin OmpF.](/cms/asset/872a9a4b-23db-467a-8a2a-02ec24fe2a15/ianb19_a_11116878_uf0004_b.gif)