Abstract
Bacterial cells Nocardia tartaricans with cis-epoxysuccinate hydrolase activity were entrapped in hardened calcium pectate gel by a commercial high performance encapsulator. This enzyme (in a single step reaction with no formation of side products) was used to hydrolyze disodium cis-epoxysuccinate to a pure enantiomer—disodium l-(+)-tartrate. Activities of this enzyme were determined using flow calorimetry. The validity of this method was corroborated by HPLC and isotachophoresis. The immobilized biocatalyst has activity (75.8 U/mgdry) able to convert disodium cis-epoxysuccinate to disodium tartrate at 94% yield in 5.5 h. Immobilization of N. tartaricans in hardened calcium pectate gel beads had a positive effect on the activity of cis-epoxysuccinate hydrolase, storage stability, yield, and time of bioconversion.
Introduction
Biotechnological preparations of organic acids have superceded chemical synthesis due to ecological and economical advantages. Those preparations are fixed for the production of pure enantiomers (l-tartaric acid, l-malic acid). l(+)-Tartaric acid is used as a food additive, in the pharmaceutical industry and viticulture (Gry and Larsen, Citation[[1978]]). Nocardia tartaricans (Miura et al., Citation[[1977]]) is the bacteria primarily used in the preparation of l-tartaric acid from cis-epoxysuccinic acid. Other bacteria from the species Corynobacterium (Zhang and Qian, Citation[[2000]]), Rhizobium, and Pseudomonas (Kamatani and Ogino, Citation[[1977]]) that contain the enzyme cis-epoxysuccinate hydrolase are also used. This enzyme hydrolyzes the substrate cis-epoxysuccinic acid on the l(+)-tartaric acid enantiomer only, in a single step reaction. Despite the attractiveness of epoxide hydrolases, cis-epoxysuccinate hydrolase has not been isolated or analyzed by modern enzymatic methods. The epoxide hydrolase (acting on 2-methyl-2-pentyloxirane or (±)-cis-2-heptene oxide) was isolated from Nocardia sp. EH1 in low yield and the partially purified enzyme proved to be considerably less stable (Kroutil et al., Citation[[1998]]). Therefore, the microbial production of l-tartaric acid still requires the use of N. tartaricans cells, and not the pure enzyme, cis-epoxysuccinate hydrolase. Immobilized cells have been prepared using a wide selection of materials (Kurillová et al., Citation[[2000]]; Rosenberg, et al., Citation[[1999]]; Zhang and Qian, Citation[[2000]]). Nocardia tartaricans entrapped in hardened calcium pectate gel (CPG) may be used for the long-term production (360 days) of the strong sequestering reagent l-tartaric acid at high concentration (1 M) (Kurillová et al., Citation[[2000]]).
The production of l-tartaric acid via the action of immobilized N. tartaricans using cis-epoxysuccinic acid as the substrate, in batch mode, has been monitored by isotachophoresis (Kurillová et al., Citation[[2000]]; Rosenberg et al., Citation[[1999]]), VIS-spectrophotometry (Kamatani and Ogino, Citation[[1977]]; Miura et al., Citation[[1977]]), and HPLC (Lian et al., Citation[[1999]]). It has been shown that flow calorimetry (FC), the so-called enzyme thermistor, can be used for the direct measurement of the catalytic properties of immobilized cells, for example, Trigonopsis variabilis with cephalosporin C-transforming activity (Gemeiner et al., Citation[[1993]]; Vikartovská-Welwardová et al., Citation[[1999]]). Due to its general detection principle (heat of reaction), FC can be a useful, versatile and standard method for characterizing various types of immobilized biocatalysts.
The process of cis-epoxysuccinate to l-tartrate conversion using cis-epoxysuccinate hydrolase, can be used as a model for flow microcalorimetry. The main advantages of this process are: highly selective biotransformation; single step reaction; conversion run without the formation of side products; high yield of tartaric acid (90–97%) (Miura et al., Citation[[1977]]; Rosenberg et al., Citation[[1999]]) and low molecular substrate. Previously, FC was used for the investigation of the kinetics of immobilized invertase (Štefuca et al., Citation[[1997]]), d-amino acid oxidase (Gemeiner et al., Citation[[1993]]) and glucoamylase (Štefuca et al., Citation[[2000]]). These enzyme catalyzed reactions were not accompanied with all the above mentioned benefits: invertase—high substrate inhibition; d-amino acid oxidase—multiple step reaction, problems with deleterious hydrogen peroxide as a subproduct; glucoamylase-high molecular substrate, substrate inhibition; contrary to l-tartaric acid microbial production. It is therefore advantageous to use FC for the determination of immobilized cis-epoxysuccinate hydrolase activity from Nocardia tartaricans.
The aim of the study was to show the positive effect of immobilization of N. tartaricans cells with hardened CPG, upon the activity of the enzyme cis-epoxysuccinate hydrolase. This study also deals with a possibility of direct measurement of the catalytic properties of bacterial cells N. tartaricans entrapped in hardened CPG using FC method.
Materials and Methods
Microorganisms and Cultivation Conditions
Nocardia tartaricans ATCC 31191 cells were grown aerobically at 30°C for 24 h in 50 mL medium containing 0.3% (w/v) meat extract (Fluka), 1% (w/v) peptone (Fluka), 0.3% (w/v) NaCl, 0.3% (w/v) KH2PO4, 0.2% (w/v) MgSO4 · 7H2O, 0.001% (w/v) FeSO4 · 7H2O, 0.003% (w/v) CaCl2 · 2H2O, 0.003% (w/v) MnSO4 · 4H2O. The medium was placed into 100 mL flasks and 1% (w/v) sodium cis-epoxysuccinate (pH 7.65) was added to the culture medium. After cultivation cells were collected by centrifugation, washed with distilled water (15 L) and stored at 4°C.
Chemicals
Potassium pectate (intrinsic viscosity [η] = 178 mL/g; dry weight = 86.06% and content of d-galacturonic acid (potassium form) = 75.90%) was prepared from commercial apple pectin (Pectin-Fabrik, Smiřice, Czech Republic) and characterized as reported previously (Institute of Chemistry, SAS, http://www.saccharides.sk/products/catalog.pdf). A 5.2% (w/w) solution of potassium pectate was prepared (dynamic viscosity 54 mPa s at 25°C, Brookfield DV-II+ viscometer) by dissolving potassium pectate in distilled water at ambient temperature. Polyethyleneimine, under trade name Sedipur CL 930, BASF (Ludwigshafen, Germany), glutaraldehyde, 50% aqueous solution was supplied from Fluka (Buchs, Switzerland). Disodium cis-epoxysuccinate was prepared from maleic anhydride and hydrogen peroxide under catalysis of Na2WO4 (Miková et al., Citation[[1998]]). Purity of the substrate was probed by Finnigan MAT SSQ 710 mass spectrometer (San Jose, USA). Electron ionization mass spectra were obtained at 200°C with direct inlet probe, 70 eV, and ionization current 200 µA.
Immobilization Procedure
A paste of N. tartaricans biomass (440 g, dry weight 23.1% w/w) was added to 3 L of distilled water. This suspension was then added to a solution of 625 g of potassium pectate in 9 L of distilled water, and filtered twice through the stainless steel sieve (56 µm pore size) using a vacuum. The standard particles were formed from aqueous pectate solution using Encapsulator Var E (open 13 nozzle system with feed pump, Nisco Engineering AG, Zürich, Switzerland). The feed pump (40 rotations per minute) produced a laminar jet of aqueous pectate solution (8.2 L/h) which was broken-up by vibrations of the electromagnetic vibrator (200 Hz) in a pulsation chamber followed by passing through 13 nozzles (300 μm in diameter) into 12 L CaCl2 solution (0.1 M) and incubated in the solution for 1 h. The CPG beads were stabilized by subsequent treatment with 2% (v/v) polyethyleneimine (12 h, 50 L) in distilled water and 1% (v/v) glutaraldehyde (1 min, 30 L) as reported previously (Kurillová et al., Citation[[1992]]). Finally the hardened beads (mean diameter: 0.9 mm) were washed with distilled water and stored in 1 M disodium cis-epoxysuccinate at room temperature.
Fermentation Conditions
The conversion in batch-wise mode was carried out in 10 mL flasks in a shaking water bath (Julabo Labortechnik GmbH, Germany) at 30°C. The hardened CPG beads with immobilized N. tartaricans (1.6 g of wet weight), and respectively intact cells (0.1 g of wet weight), and 4.9 ml disodium cis-epoxysuccinate (1 M, pH 7.6), and 0.1 mL sodium deoxycholate (0.02% w/v) were used for the bioconversion to disodium l-tartrate. During bioconversion (5.5–20 h), samples were taken to measure the degree of conversion and determination of catalytic activity of cis-epoxysuccinate hydrolase using HPLC and isotachophoresis.
Analytical Methods
Optical Rotation
Optical rotations were measured with a Perkin-Elmer Model 141 polarimeter at 20°C (C = 10) in 1 mL cell.
Determination of Enzyme Activity
The activities of cis-epoxysuccinate hydrolase were determined using HPLC, isotachophoresis, and flow calorimetry.
One unit of activity of cis-epoxysuccinate hydrolase was defined as the amount of enzyme capable of generating 1 µmol of disodium tartrate per hour under experimental conditions.
Flow Calorimetry (FC)
Flow calorimeter (3300 Thermal Assay Probe, Advanced Biosensor Technology, AB, Lund, Sweden) and the procedure have been described elsewhere (Gemeiner et al., Citation[[1993]]; Štefuca et al., Citation[[1997]], Citation[[2000]]). The main part of the system is a thermostatic cell with immobilized enzyme/cells column. The column is operated as a small packed bed reactor. The temperature difference between the column input and output, ΔT is measured by thermistor. The column (7 mm i.d. × 20 mm) was packed with wet immobilized bacterial cells (approx. 500 mg of wet weight, which corresponds to a dry mass of immobilized cells ranging from 3.8 to 4.2 mg). Tris–HCl buffer (50 mM, pH 7.6) was passed through the system at 1 mL/min. The buffered disodium cis-epoxysuccinate (1 M) after thermal equilibration (30°C) was passed continuously through the FC column until steady heat production was obtained.
Two measurements techniques of FC were applied ():
Single flow mode
Mode with total recirculation
Figure 1. The experimental set-up of calorimetric measurements (flow mode and mode with total recirculation of the reaction mixture) of activity of immobilized biocatalysts.
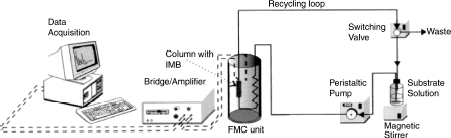
In the single flow mode (switching valve opened to waste), disodium cis-epoxysuccinate (1 M, pH 7.6) after thermal equilibration (30°C) was passed continuously through the FC column till steady-state production was achieved. The temperature change due to the hydrolytic reaction was calculated from the height of the thermogram (Gemeiner et al., Citation[[1993]]; Vikartovská-Welwardová et al., Citation[[1999]]). In the recirculation mode (closed waste) the valve from waste was switched to the recirculation loop returning the reaction mixture to a stirred reservoir. FC is adapted to the differential reactor system with infinite flow recirculation (Štefuca et al., Citation[[1997]]; Citation[[2000]]). The disodium tartrate concentration (product of the hydrolysis disodium cis-epoxysuccinate using immobilized N. tartaricans with cis-epoxysuccinate hydrolase activity) in the samples (50 µL) withdrawn from the stirred reservoir (2.57 mL and the total liquid volume in the system was 5.57 mL) was analyzed using HPLC.
HPLC
The HPLC was performed using a Shimadzu liquid chromatograph model LC-10 AD furnished with a SPD-10AV Shimadzu UV–VIS detector operating at 210 nm and Shimadzu Class VP software. The system was fitted with a glass column (300 × 3.3 mm) packed with Separon SGX C18, 7 µm (Tessek Ltd, Prague) at room temperature. The aqueous mobile phase was adjusted to pH 2.03 with perchloric acid. The flow rate was 1 mL/min. Samples (20 µL) diluted (980 times) with mobile phase were injected. Calibration with reference samples gave the following retention times (min): 2.09–2.13 for disodium l-(+)-tartrate and 2.21–2.24 for disodium cis-epoxysuccinate, respectively (Lian et al., Citation[[1999]]).
The activities of cis-epoxysuccinate hydrolase were determined by height comparison of the peaks of disodium l-(+)-tartrate of the sample from bioconversion on RP-HPLC chromatogram against the reference samples. The time of total conversion of disodium cis-epoxysuccinate to l-(+)-tartaric acid disodium salt was determined as the time of sample taken from bioconversion when the signal of substrate on RP-HPLC chromatogram was absent and only the product peak visible.
Isotachophoresis
Tartaric and cis-epoxysuccinic acids were determined by isotachophoresis using a column-coupling isotachophoretic analyzer (model ZKI, IRANT, Slovak Republic) (Polonský et al., Citation[[1985]]). The following electrolytes were used: Leading-10 mM hydrochloric acid, β-alanine, 0.1% methyl hydroxyethyl cellulose; terminating-5 mM acid.
Results and Discussion
Purity of the substrate is very important, and it is essential that disodium cis-epoxysuccinate contains no residue of maleic and tartaric acid racemate. The preparation succeeded and this was verified by mass spectrometry, HPLC, and optical rotation (Pätoprstý et al., in preparation).
The next stage in the production of l-tartaric acid is the preparation of N. tartaricans biomass with the highest activity of cis-epoxysuccinate hydrolase. The biomass was obtained with the activity ca. 30 U/mgdry, significantly greater than described previously (Rosenberg et al., Citation[[1999]]).
Previous results show that hardened CPG (stabilized by the combined treatment of polyethyleneimine and glutaraldehyde) comprising N. tartaricans resisted, for a long time (360 days), the destructive effects of the product (strong sequestering reagent l-(+)-tartaric acid) at high concentration (1 M) (Kurillová et al., Citation[[2000]]). Nonhardened CPG (without a two-stage crosslinking procedure with polyethyleneimine and glutaraldehyde) was destroyed after 21 h. The reference materials, hardened and nonhardened calcium alginate gels, were destroyed over 3 h and 30 min, respectively. This information was utilized during the immobilization of N. tartaricans in hardened CPG by a commercial high capacity encapsulating apparatus-13 nozzle Encapsulator Var E with feed pump (from Nisco Engineering AG Zürich). The average productivity per nozzle is 400 mL/h whereas this can significantly differ in function of the nozzle diameter and jet speed.
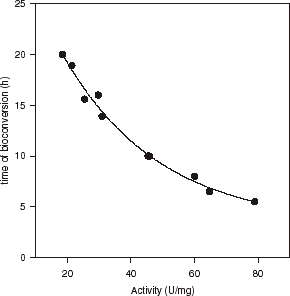
The cells of N. tartaricans (30 U/mgdry) in different quantity (6–8.7 mg dry cells/1 g gel) were entrapped to the apple CPG and were stabilized by polyethyleneimine and glutaraldehyde. A two stage crosslinking procedure led to the formation of a more compact layer on the bead surface (Kurillová et al., Citation[[1992]], Citation[[2000]]). Using scanning and transmission electron microscopy, it was confirmed that hardening/stabilization of CPG by chemical crosslinking changes only the texture of the bead surface (Kurillová et al., Citation[[2000]]). The stabilization procedure did not significantly change gel bead interior or morphologic properties and biotransformation activity of immobilized bacterial cells against disodium cis-epoxysuccinate (Kurillová et al., Citation[[2000]]). The positive effect of the immobilization of yeasts T. variabilis (Kurillová et al., Citation[[1992]], Citation[[2000]]), and Kluyveromyces marxianus (Tomáška et al., Citation[[1995]]) with hardened CPG upon the activity of the d-amino acid oxidase, respectively β-galactosidase was demonstrated. An exponential decay (r2 = 0.987) was found when time of total conversion of disodium cis-epoxysuccinate to l-(+)-tartaric acid disodium salt was plotted against the activities of cis-epoxysuccinate hydrolase in immobilized bacterial cells (N. tartaricans) ().
Figure 2. Exponential decay (three parameter single: y = y0 + ae−bx; y0 = 3.22, a = 30.94, b = 0.033) of total bioconversion time of disodium cis-epoxysuccinate to disodium tartrate as a function of activity of cis-epoxysuccinate hydrolase in cells N. tartaricans immobilized in CPG beads. Conditions of bioconversion: 1 M disodium cis-epoxysuccinate (pH 7.6), 1% (w/v) sodium deoxycholate, temperature 30°C, stirring at 130 rpm, concentration of biomass 6–8.7 mg (dry weight)/1 g gel.
Stereospecific hydrolysis of 1 M cis-epoxysuccinate leads to the formation of l-(+)-tartrate pure enantiomer catalyzed by cis-epoxysuccinate hydrolase in batch-wise mode. The conversion yield (94%) was obtained from optical rotation of the product after total conversion , divided by the optical rotation of reference sample
. The formation of byproducts was not detected during conversions.
The immobilized N. tartaricans cells were stored in 1 M disodium cis-epoxysuccinate that preserved consecutive cis-epoxysuccinate hydrolase activity induction and cells permeabilization (). This had an impact on the activity and bioconversion time. In hardened CPG immobilized cells (8.4 mg dry weight/1 g gel), during the day of preparation, the activity was 21.8 U/mg with a conversion time of 18 h. After 7 days the activity was 60.1 U/mg and the conversion time shortened to 8 h and after 90 days the activity raised to 79 U/mg with a conversion time of only 5.5 h. The storage stability of this preparation was measured under batch flow conditions. The activity was determined by various analytical methods: HPLC, FC, and isotachophoresis (). There was no significant difference between these monitoring techniques. Due to its versatility, rapidity, and simplicity, flow calorimetry was chosen for the determination of catalytic activity of cis-epoxysuccinate hydrolase in N. tartaricans entrapped in hardened CPG.
Table 1. Storage stability of cis-epoxysuccinate hydrolase in N. tartaricans (8.4 mg dry weight/g CPG) immobilized in hardened CPG measured by stirred or flow conditions using HPLC, isotachophoresis, respectively FC method
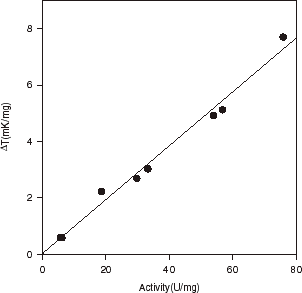
With the single flow mode technique of flow calorimeter (), steady-state temperature signals (ΔT ) were measured. The validity of this procedure was verified by comparison with an independent HPLC method, in which the immobilized bacterial cells were assessed under stirred batch reactor conditions (). Good agreement was found between the methods: the correlation coefficient (r2) was 0.990 (). The catalytic activity of hardened CPG with N. tartaricans can be directly compared using the thermometric value of ΔT (at 1 M substrate).
Figure 3. A linear correlation between activities of cis-epoxysuccinate hydrolase (in N. tartaricans), as obtained by HPLC from stirred batch reactor, and by flow calorimetry. Activity measured by HPLC was expressed in units (U) per weight (mg) of the dry entrapped biocatalyst; activity measured by FC in ΔT (mK/mg).
In recirculation mode (), the enzyme thermistor is adapted to the differential reactor system with infinite flow recirculation (Štefuca et al., Citation[[1997]], Citation[[2000]]). This was obtained by switching the valve from waste to the recirculation loop thus returning the reaction mixture to a stirred reservoir. The system is closed, and the steady-state cannot be reached because of continual consumption of substrate in the enzyme reactor. This configuration of FC has successfully been applied to a kinetic study of invertase immobilized by specific binding on Concanavalin A-cellulose conjugates (Štefuca et al., Citation[[1997]]), and respectively for glucoamylase covalently bound to controlled-pore glass particles (Štefuca et al., Citation[[2000]]). In this case we exploited this procedure to monitor the storage stability () of the enzyme cis-epoxysuccinate hydrolase in N. tartaricans immobilized in hardened calcium pectate gel. The disodium tartrate samples were withdrawn from the stirred reservoir and then analyzed using HPLC. The acquired activities were not greatly different (approx. 15%) from these values obtained with stirred batch reactor conditions by analyzed HPLC, FC, or isotachophoresis methods ().
Conclusion
It was shown, that immobilization of N. tartaricans bacterial cells in hardened CPG has a positive effect on the activity of cis-epoxysuccinate hydrolase (higher activity, good storage stability, good mechanical properties, and low time of bioconversion). Our results demonstrate that the FC technique provides a fast and simple determination of the activity of cis-epoxysuccinate hydrolase in N. tartaricans entrapped in hardened pectate gel.
Acknowledgment
This work was supported in part the Slovak Grant Agency for Science “VEGA” (grant No. 1/0067/03).
References
- Gemeiner P., Štefuca V., Welwardová A., Michalková E., Welward L., Kurillová L.', Danielsson B. Direct determination of the cephalosporin transforming activity of immobilized cells with use of an enzyme thermistor. 1. Verification of the mathematical model. Enzyme Microb. Technol. 1993; 15: 50–56
- Gry J., Larsen J. C. Metabolism of L(+) and D(−)-tartaric acids in different animal species. Arch. Toxicol. Suppl. 1978; 1: 351–353, [PUBMED], [INFOTRIEVE]
- http://www.saccharides.sk/products/catalog.pdf, Institute of Chemistry-SAS: List of products
- Kamatani Y., Ogino K.. Production of L(+)-tartaric acid. US Patent 4,011,135, 1977
- Kroutil W., Genzel Y., Pietzsch M., Syldatk Ch., Faber K. Purification and characterization of highly selective epoxide hydrolase from Nocardia sp. EH1. J. Biotechnol. 1998; 61: 143–150, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kurillová L'., Gemeiner P., Ilavský M., Štefuca V., Polakovič M., Welwardová A., Tóth D. Calcium pectate gel beads for cell entrapment. 4. Properties of stabilized and hardened calcium pectate gel beads with and without cells. Biotechnol. Appl. Biochem 1992; 16: 236–251
- Kurillová L'., Gemeiner P., Vikartovská A., Miková H., Rosenberg M., Ilavský M. Calcium pectate gel beads for cell entrapment. 6. Morphology of stabilized and hardened calcium pectate gel beads with cells for immobilized biotechnology. J. Microencapsulation 2000; 17: 279–296, [CROSSREF], [CSA]
- Lian H. Z., Mao L., Ye X. L., Miao J. Simultaneous determination of oxalic, fumaric, maleic and succinic acids in tartaric and malic acids for pharmaceutical use by ion-suppression reversed-phase high performance liquid chromatography. J. Pharm. Biomed. Anal. 1999; 19: 621–625, [CROSSREF], [CSA]
- Miková H., Rosenberg M., Liptaj T. Influence of molybdate and tungstate ions on activity of cis-epoxysuccinate hydrolase in Nocardia tartaricans. Biotechnol. Lett. 1998; 20: 833–835, [CROSSREF], [CSA]
- Miura Y., Yutami K., Izumi Y.. Process for preparing L-tartaric acid. US Patent. 4,010,072, 1977
- Pätoprstý V., Bučko M., Vikartovská A., Nahálka J., Gemeiner P. Monitoring of bioconversion cis-epoxysuccinate to tartrate by Nocardia tartaricans. Anal. Chem. 2004, (to be submitted)
- Polonský J., Repášová L'., Pekarovičová A., Košík M. Isotachophoretic analysis of saccharidic acids. J. Chromatogr. 1985; 320: 111–118, [CROSSREF]
- Rosenberg M., Miková H., Krištofíková L'. Production of L-tartaric acid by immobilized bacterial cells Nocardia tartaricans. Biotechnol. Lett. 1999; 21: 491–495, [CROSSREF], [CSA]
- Štefuca V., Čipáková I., Gemeiner P. Investigation of immobilized glucoamylase kinetics by flow calorimetry. Themochimica Acta 2000; 378: 79–85, [CSA]
- Štefuca V., Welwardová A., Gemeiner P. Flow microcalorimeter auto-calibration for the analysis of immobilized enzyme kinetics. Anal. Chim. Acta 1997; 355: 63–67, [CROSSREF]
- Tomáška M., Gemeiner P., Materlín I., Šturdík E., Handríková G. Calcium pectate gel beads for cell entrapment: A study on the stability of Kluyveromyces marxianus whole-cell lactase entrapped in hardened calcium pectate and calcium alginate gels. Biotechnol. Appl. Biochem. 1995; 21: 347–356, [CSA]
- Vikartovská-Welwardová A., Michalková E., Gemeiner P., Welward L. Stabilization of D-amino-acid-oxidase from Trigonopsis variabilis by manganese dioxide. Folia Microbiol. 1999; 44: 380–384, [CSA]
- Zhang J. G., Qian Y. J. Production of L(+)-tartaric acid by immobilized Corynebacterium sp. JZ-1. Sheng Wu Gong Cheng Xue Bao 2000; 16: 188–192, [PUBMED], [INFOTRIEVE], [CSA]