Abstract
Our previous studies have indicated that encapsulated tyrosinase and crosslinked hemoglobin with tyrosinase (polyhemoglobin–tyrosinase) decrease systemic tyrosine level significantly in rats. However, we need a few days of oral administration of encapsulated tyrosinase before the systemic tyrosine level starts to decrease. Although intravenous injection of polyhemoglobin–tyrosinase can lower the systemic tyrosine to about 10% within an hour, the level increases towards normal after 24 h. We therefore investigate the effects of intravenous injection of polyhemoglobin–tyrosinase combined with oral administration of encapsulated tyrosinase on lowering the systemic tyrosine level. In addition, we further optimize this combined method for lowering systemic tyrosine in animal studies and have found out that two intravenous injections of polyhemoglobin–tyrosinase followed by three times a day oral administration of encapsulated tyrosinase could immediately lower the body tyrosine and maintain this low level as long as the oral administration is continued.
Introduction
Melanoma, a malignant neoplasm derived from melanocytes of the skin and other sites, continues to increase in frequency worldwide. In the United States and Canada, the rate of increase of melanoma is greater than for any other tumors except lung cancer in women, and about 20–25% of patients with melanoma die from metastasis (Charles, Citation[[2001]]).
Many different methods are explored for the treatment of melanoma. Surgery to remove the tumor plus regional lymphadenectomy is at present an effective therapy for primary melanoma (Gennari et al., Citation[[2000]]; Roses et al., Citation[[1985]]; Veronesi and Cascinelli, Citation[[1991]]; Wong et al., Citation[[1987]], Citation[[2000]]). Radiation therapy plays an important role in the management of melanoma and following therapeutic lymph node dissection for bulky disease (Cocconi et al., Citation[[1992]]). In chemotherapy, Dacarbazine (DTIC) has been the most extensive clinical trials of any single agent with a reported response rate of 22% (Serrone et al., Citation[[2000]]). Nitrosoureas and Cisplatin are other single agents that have undergone adequate clinical trials (Bezwoda, Citation[[1997]]). Hormones have also been suggested (Pavey and Gabrielli, Citation[[2002]]). Others include combination chemotherapy, adjuvant chemotherapy, regional perfusion and high-dose chemotherapy with autologous bone marrow transplantation although most combination chemotherapy is usually more toxic (Abdi et al., Citation[[1987]]; Cocconi et al., Citation[[1992]]; Fraker, Citation[[1999]]; Meisenberg, Citation[[1996]]; Wolff et al., Citation[[1989]]). Immunotherapy including interferons, interleukin-2, TNF and many other biologic agents have been studied extensively in the treatment of melanoma (Quan and Mitchell, Citation[[1993]]; Rosenberg et al., Citation[[1993]]). Recently, as the lack of effective treatment for this advanced disease, gene therapy of melanoma has been investigated in the treatment of melanoma (Rosenberg et al., Citation[[1996]]).
However, at present there is no practical treatment for malignant melanoma. Another approach is to lower systemic tyrosine by dietary restriction of tyrosine because the growth of melanoma cells needs higher level of tyrosine than that of normal melanocytes (Ge et al., Citation[[2002]]; Meadows et al., Citation[[2001]]). In fact, high cellular intake of tyrosine is increased further by the requirement of this amino acid for protein synthesis in the malignant melanoma cells. The rationale for dietary restriction is to limit tyrosine availability for protein synthesis and tumor growth. Another research group has investigated the use of dietary restriction in tyrosine and phenylalanine for the treatment of the acute and chronic forms in the diseases of the disorders of tyrosine oxidation (Halvorsen and Gjessing, Citation[[1964]]). It benefited to most patients and associated with dramatic clinical improvement. Demopoulos (Citation[[1966]]) later showed that this approach can be useful for patients with advanced malignant melanoma. However, this regimen is cumbersome and complex. It is appetite depression due to not palatable and causes nausea and vomiting, thus making it not practical. Another disadvantage for this nutritional control of tumor growth is severe body weight loss (Meadows and Oeser, Citation[[1983]]).
Therefore, we design a novel method of combining artificial cells containing tyrosinase and polyhemoglobin–tyrosinase (PolyHb–tyrosinase) to lower systemic tyrosine level. Our previous studies have shown that encapsulated tyrosinase effectively decrease tyrosine level in vitro (Yu and Chang, Citation[[2002]]). Further in vivo research shows that oral administration of artificial cells encapsulated with tyrosinase could decrease tyrosine level although it takes a few days to reach a low level (Yu and Chang, Citation[[2004]]). We also find that intravenous injection of PolyHb-tyrosinase could lower systemic tyrosine level within an hour. However, the lowered tyrosine level increases towards normal level after 24 h. We therefore combine the oral administration method with intravenous injection to lower systemic tyrosine level and keep it at that low level as long as the oral administration is continued. We further optimize this combined method for its efficiency in animal experiments.
Methods
Materials
l-Tyrosine (98% TLC), tyrosinase from mushroom (EC 1.14.18.1, 5350 units/mg stated activity), hemoglobin from bovine (lyophilized powder), l-lysine (monohydrochloride, Sigma Ultra >99%) were purchased from Sigma-Aldrich (Ontario, Canada). Glutaraldehyde (25%) was obtained from BDH (Toronto, Ontario, Canada). Collodion was purchased from Fisher Scientific (Ontario, Canada). Purified bovine hemoglobin (10 g/dL) was purchased from Biopure Corporation (Boston, MA, USA). All other reagents were of analytical grade.
Preparation of PolyHb and PolyHb-tyrosinase
Polyhemoglobin (PolyHb) solution was prepared by an updated method (Chang, Citation[[1997]]). This was carried out in a cold room at 4°C. First, 1.3 M lysine at a molar ratio of 7:1 lysine/hemoglobin was added to hemoglobin solution. The mixture was left rotating in a shaker at speed of 150 rpm in the cold room for an hour. In the preparation of PolyHb mixtures, an equivalent volume of buffer replaced enzyme condition. Next, 0.5 M glutaraldehyde was added at a molar ratio of 16:1 glutaraldehyde/hemoglobin. After 24 h under aerobic conditions with constant stirring at 4°C, the reaction was stopped with 2.0 M lysine at a molar ratio of 200:1 lysine/hemoglobin. In the preparation of polyhemoglobin–tyrosinase (PolyHb–tyrosinase), reaction mixtures were prepared containing hemoglobin (10 g/dL), tyrosinase (6000 U/mL) in 0.1 M potassium phosphate buffer, pH 7.6. Prior to the start of crosslinking, 1.3 M lysine was added at a molar ratio of 7:1 lysine/hemoglobin. Crosslinking reaction was started with the addition of glutaraldehyde at a molar ratio of 16:1 glutaraldehyde/hemoglobin. Glutaraldehyde was added in four equal aliquots over a period of 15 min. After 24 h under aerobic conditions with constant stirring at 4°C, the reaction was stopped with 2.0 M lysine at a molar ratio of 200:1 lysine/hemoglobin. Solutions were dialyzed in physiological saline solution overnight and passed through sterile 0.45 µM filter. Aliquots (500 µL) of crosslinked preparation were concentrated using 100 KD microconcentrators (Amicon, Beverly, MA, USA). Samples were centrifuged at 2500 g for 55 min at 23°C. Then, retentate was collected. Hemoglobin concentration was determined by cyanomethemoglobin at 540 nm according to “Total Hemoglobin Kit” from Sigma-Aldrich (Ontario, Canada). Final retentates were stored at 4°C until use.
Determination of Oxygen Affinity of Hemoglobin and PolyHb–tyrosinase
The oxygen dissociation curve for hemoglobin and PolyHb–tyrosinase were determined by the TCS Hemoxanalyser (TCS Medical Products Co., USA). Samples (5 mL) containing 0.3 g/dL of hemoglobin or 24 h crosslinked PolyHb-tyrosinase in 37°C 0.1 M PBS at pH 7.4 were used to obtain hemoglobin oxygen dissociation curves.
Preparation of Control Artificial Cells
Control artificial cells were prepared by an updated method (Chang, Citation[[1972]], Citation[[1985]]; Chang and Prakash, Citation[[2001]]). One gram of hemoglobin and 200 mg Tris were dissolved in 10 mL double distilled deionized water. Stirred with a metal rod until everything was dissolved. Gravity filtered the solution through a Waterman #42 filter into an Erlenmeyer flask. Two and a half milliliters of this 10 g/dL hemoglobin solution was added to a 150 mL glass beaker and 25 mL of water-saturated ether was added. The mixture was immediately stirred with a Fisher Jambo magnetic stirrer at a setting of 5 for 5 s. While stirring was continued, 25 mL of a cellulose nitrate solution was added. Stirring was continued for another 60 s. The beaker was covered and allowed to stand unstirred at 4°C for 45 min. The supernatant was decanted and 30 mL of n-butyl benzoate was added. The mixture was stirred for 30 s at the same magnetic stirrer setting. The beaker was allowed to stand uncovered and unstirred at 4°C for 30 min. Then the butyl benzoate was removed completely after centrifugation at 350 g for 5 min. Twenty five milliliters of the Tween 20 at 50% (v/v) concentration was added. Stirring was started at a setting of 10 for 30 s. Next, 25 mL of water was added and stirring continued at a setting of 5 for 30 s, then 200 mL of water was added. The supernatant was removed and the microcapsules were washed three more times by 1% Tween 20. The microcapsules were then suspended in 1% Tween 20 at 4°C until use. In properly prepared microcapsules, there should be no leakage of hemoglobin after the preparation.
Preparation of Tyrosinase Loaded Microcapsules
1.907 milligram of 5350 units/mg tyrosinase were dissolved in 5 mL of 10% hemoglobin solution, and the final pH was adjusted to pH 8.5 with Tris buffer. Then, followed the methods described above to immobilize tyrosinase in collodion membrane microcapsules. Microcapsules prepared as a 50% suspension for later feeding. Tyrosinase loaded microcapsules were administered orally to test group. All artificial cells were prepared daily and stored in 1% v/v Tween 20 solution at 4°C until use. Before oral administration for both control group and test group, artificial cells suspended in Tween 20 were washed and resuspended in 0.1 M pH 8.5 Tris–HCl buffer. The total volume of artificial cells for feeding was 2.5 mL (1 mL artificial cells plus 1.5 mL Tris–HCl buffer). This buffer was enough to protect the microencapsulated tyrosinase activity during its passage through the stomach with its acidic medium. For oral feeding, artificial cells without enzyme (control artificial cells) were fed to the control group. In the test group, artificial cells containing tyrosinase were given.
Animal Studies
Fasted male Sprague–Dawley rats (130–150 g) were purchased from Charles River (St-Constant, Quebec, Canada). They were kept in a controlled 12-h light/dark environment with food and water ad libitum. All rats were acclimatized for atleast 3 days prior to use. All animal experiments were performed according to the regulations of McGill University on Animal Care. In this study, two animal groups (five rats in each group) were studied: (1) control group: fed with artificial cells without enzyme; (2) test group: fed with artificial cells loaded with tyrosinase. Each experiment began on day 0 with blood taken, and no artificial cells were administered or intravenous injection was done on that day.
Intravenous Injection
In the case of intravenous injection, we cannulated the femoral vein and artery of rat as follows. The rats were anaesthetized with intraperitoneal injection of pentobarbital (65 mg/kg, Somnotol, Decton Dickinson, NJ, USA). Polyethylene cannulae were inserted and secured distal to the superficial epigastric branches in the femoral arteries (PE-10, Clay Adams) and veins (PE-50, Clay Adams). Proper vessel access was tested with a small volume injection of heparinized saline (50 IU/mL). Samples were injected through femoral vein, and blood was taken from femoral artery. The plasma in each blood sample was separated from the blood by centrifuge and placed in a 1.5 mL plastic tube, then stored at −80°C until analyzed. The tyrosine concentration in plasma was analyzed by fluorometric method using Perkin Elmer Luminescence Spectrometer LS50B (Waalkes and Udenferiend, Citation[[1957]]).
Oral Administration of Artificial Cells Three Times Per Day
On day 0, took blood at 4:00 p.m. On the next day, oral administration of artificial cells three times at 10:00 a.m., 2:00 p.m., and 6:00 p.m. Blood sample was take every day just after the second feeding. The plasma in each blood sample was separated from the blood by centrifuge and placed in a 1.5 mL plastic tube, then stored at −80°C until analyzed.
Three Oral Doses Per Day Combined with One Intravenous Injection on Day 1
On day 0, took blood at 4:00 p.m. No artificial cells were administrated on this day. From that day on, and every subsequent day for 4 days, artificial cells were administrated orally at 10:00 a.m., 2:00 p.m., and 6:00 p.m., blood samples were taken every day just after the second feeding. On day 1, PolyHb solution was injected to the control group and PolyHb-tyrosinase solution to the test group at volume of 1 mL per 250 g body weight. Body weights for all rats were monitored everyday.
Three Oral Doses Per Day Combined with Two Intravenous Injections on Day 1 and Day 2
On day 0, took blood at 4:00 p.m. No artificial cells were administrated on this day. From that day on, and every subsequent day for 4 days, artificial cells were administrated orally at 10:00 a.m., 2:00 p.m., and 6:00 p.m. and took blood samples every day just after the second feeding. On day 1, PolyHb solution was injected to control group and PolyHb–tyrosinase solution to test group at volume of 1 mL per 250 g body weight. On day 2, half volume of PolyHb or PolyHb-tyrosinase solution was injected to the control group and the test group respectively. Body weights for all rats were monitored everyday.
Statistical Analysis
The differences of tyrosine level in rat's plasma between the control group and the test group at the same time point were determined by using Student's t-test within ANOVA and considered significant at p<0.05.
Results and discussions
Effects of Intravenous Injection on Lowering Systemic Tyrosine
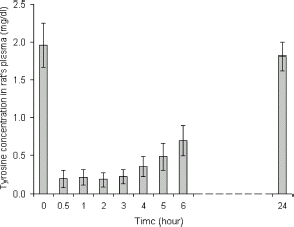
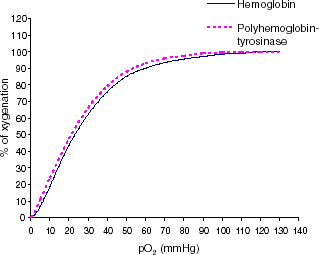
We first investigate the effect of intravenous injection of PolyHb–tyrosinase on lowering systemic tyrosine level. One injection of PolyHb–tyrosinase was given and tyrosine level was monitored every hour. From , tyrosine level markedly decreased after injection. In the first hour, systemic tyrosine decreased to about 11%. As the presence of a high concentration of oxygen is important in radiotherapy for cancer cells (Cooper, Citation[[1998]]), we further carry out the study of oxygen characteristics of PolyHb–tyrosinase. In , purified bovine hemoglobin served as the control group, there was no significant difference between polyhemoglobin-tyrosinase solution and free hemoglobin solution. Based on the analysis of oxygen saturation curves, the P50 values for noncrosslinked hemoglobin and PolyHb–tyrosinase were 23 and 21 mmHg, respectively. They possess oxygen transport characteristics similar to other crosslinked hemoglobins (Fronticelli and Bucci, Citation[[1994]]; Fronticelli et al., Citation[[1984]], Citation[[1995]]; Yu and Chang, Citation[[1996]]). Thus, polymerization process did not alter oxygenation characteristics of this modified hemoglobin. In addition to removing tyrosine, this preparation has the potential advantages of more easily perfuse the melanoma to supply more oxygen to allow for more effective chemotherapy ().
Figure 2. Oxygen dissociation curve of pure bovine hemoglobin in the free form and PolyHb–tyrosinase form. All oxygen–hemoglobin dissociation curves are presented as average of three trials.
However, tyrosine level increases towards normal level after 24 h (). Our studies in melanoma bearing mice show that daily intravenous injection is needed to maintain a low systemic tyrosine level for 19 days test period (Yu and Chang; submitted). This results in significant delay in the growth of the melanoma in the tumor bearing mice (Yu and Chang; submitted). However, prolonged intravenous injection much beyond this level may not be practical. As a result, we look at another approach that would be suitable for prolonged administration.
Effects of Oral Administration of Artificial Cells on Lowering Tyrosine Level
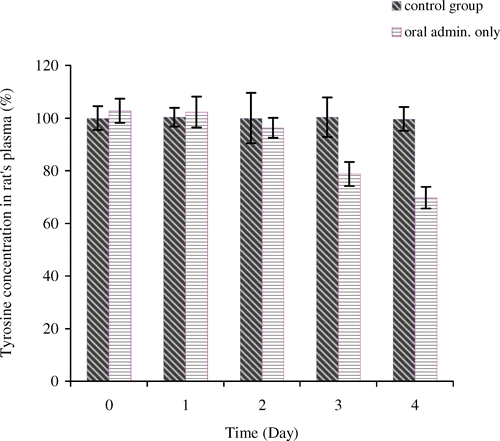
The aim of this study is to see whether oral administration of encapsulated tyrosinase can lower systemic tyrosine level within a week. Oral uses of artificial cells containing other enzymes have already been successfully demonstrated in this laboratory and offer several advantages over other methods (Bourget and Chang, Citation[[1986]], Citation[[1989]]). Based on enterorecirculation theory, the major source of intestinal amino acids comes from extensive enterorecirculation of amino acids (Chang et al., Citation[[1995]]). We therefore, use tyrosinase artificial cells to degrade specific amino acid, tyrosine, and remove this unwanted amino acid from body system. By giving artificial cells orally three times a day, we found that the systemic tyrosine level only decreased to 79 ± 5% of the control level on day 3 (). In the first two days, there was no difference in tyrosine level between the control group and the test group. Previous research has shown that 67% of tyrosine level would be needed to inhibit the growth of malignant melanoma (Meadows et al., Citation[[1982]]). However, when continue this oral administration to 22 days, the systemic tyrosine level fell to 53 ± 6% by the end of experiment (Yu and Chang, submitted). Thus, we conclude that only oral administration does not decrease the tyrosine level sufficiently at the beginning.
Three-Dose/Day Combined with One Intravenous Injection on Day 1
Since intravenous injection of PolyHb-tyrosinase decreases tyrosine level quickly, we investigate whether we could decrease tyrosine to a low level at the beginning of our experiment and keep this low level with oral administration of encapsulated tyrosinase. Body weight and tyrosine level were monitored. On day 1, after intravenous injection, tyrosine level decreased rapidly to 53 ± 4% of the control group. This is consistent to the results we obtained in our previously intravenous studies. On day 2, tyrosine level increased to 73 ± 6% of the control level. However, with oral administered of microencapsulated tyrosinase at the same time, tyrosine level started to decrease by reaching 59 ± 3% on day 4 (). Our previous results have indicated that oral administration of artificial cells alone cannot lower tyrosine level significantly or keep it at a very low level during the first 2 days of the treatment. However, the addition of one intravenous injection of PolyHb–tyrosinase to oral encapsulated tyrosinase was only able to maintain a sufficiently low tyrosine level one day after on day 1. Two days later on days 2 and 3, the tyrosine level in the test group was significantly lower than that in the control group, but it was not low enough to reach the level which would inhibit tumor growth. As a result, we added an additional intravenous injection for the next section of study. showed that the statistical studies for tyrosine level in the control group and the test group. There was no significant difference in body weight between the control group and the test group ().
Figure 4. Tyrosine concentration in rat's plasma (%). For control group: oral administration of artificial cells containing no tyrosinase three times a day with one injection of 1 mL of PolyHb solution per 250 g body weight on day 1. For test group: oral administration of artificial cells containing tyrosinase three times a day with one injection of 1 mL of PolyHb–tyrosinase solution per 250 g body weight on day 1.
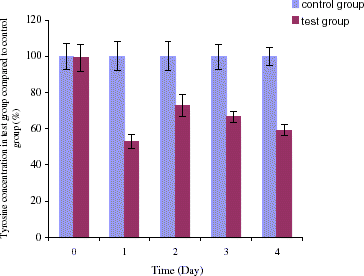
Figure 5. Body weight (gram) of rats for four days experiment. For control group: oral administration of artificial cells containing no tyrosinase three times a day with one injection of 1 mL of PolyHb solution on day 1. For test group: oral administration of artificial cells encapsulated tyrosinase three times a day with one injection of 1 mL of PolyHb–tyrosinase solution on day 1.
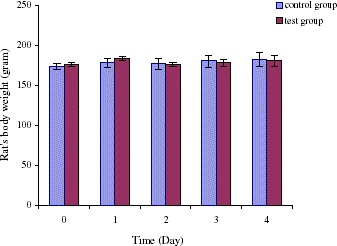
Table 1. p-Value in plasma tyrosine concentration between the control group and the group of oral administration combined with one intravenous injection
Three-Dose/Day Combined with Two Intravenous Injections on Days 1 and 2
The following experiment is designed to investigate if it is possible to maintain a sufficiently lower tyrosine level on the second day after the first intravenous injection. This is by giving a second intravenous injection on the second day that is half the volume of the first injection. In this experiment, we followed tyrosine levels in three groups including the control group, the second group in which only orally administrated encapsulated tyrosinase and the third group of two intravenous injections combined with oral administration. In the third group, on day 1 tyrosine level decreased to 54 ± 6% after the first injection. On day 2, another half volume of injection was given and the tyrosine level was maintained at a low level of 61 ± 7%. The difference between the two days after the first injection and the second injection is 7%, which is much lower than that of after one injection. On day 3, tyrosine level was 70 ± 7% and on day 4 tyrosine level was 61 ± 8% (). In the second group in which oral administration of encapsulated tyrosinase with no intravenous injection, there were no significant changes in tyrosine level until day 3. Starting at day 3, tyrosine level in this only oral feeding group was decreased to 79 ± 5%, and as this feeding continued the tyrosine level was further decreased to 70 ± 4% on day 4. There were no significant changes in tyrosine level for the control group. Taken together, comparing results from these three groups, we found that in the third group which was given combined method intravenous and oral treatment, the tyrosine level rapidly decreased starting on the first day and the level was maintained at 61 ± 7% on day 4. The second group which was only given by oral administration, the tyrosine level did not change during the first day and only reached of 70 ± 4% on day 4. Although intravenous injections could rapidly decrease the tyrosine level to below the normal concentration, it could not maintain a low level for more than one day. On the other hand, the much more convenient method of oral administration of artificial cells containing tyrosinase can bring down the tyrosine level in the body except it takes more than 3 days to be effective. Therefore, we combine these two methods. Our data have shown that the combined method could lower tyrosine level and maintain this low level. Furthermore, this novel approach is unlike the tyrosine restricted diet that causes nausea, vomiting, and severe weight loss. In the combined method there was no significant difference in body weight changes during the study (). shows the statistical analysis comparing tyrosine concentrations in the control group and the test group.
Figure 6. Tyrosine concentration in rat's plasma (%). For control group: oral administration of artificial cells containing no tyrosinase three times a day with one injection of 1 mL of PolyHb solution day 1 and another half volume injection on day 2. For group of oral administration only: oral administration of artificial cells containing tyrosinase three times a day. For test group of oral administration and i.v. injection: oral administration of artificial cells containing tyrosinase three times a day with one injection of 1 mL of PolyHb–tyrosinase solution on day 1 and another half volume injection on day 2.
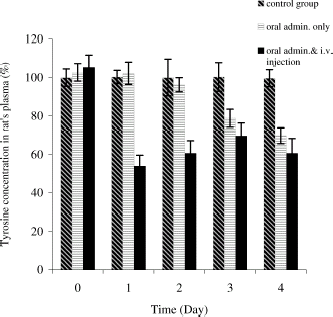
Figure 7. Body weight (gram) of rats for 4 days experiment. For control group: oral administration of artificial cells containing no tyrosinase three times a day with one injection of PolyHb solution on day 1 and another half volume injection on day 2. For test group: oral administration of encapsulated tyrosinase three times a day with one injection of PolyHb–tyrosinase solution on day 1 and another half volume injection on day 2.
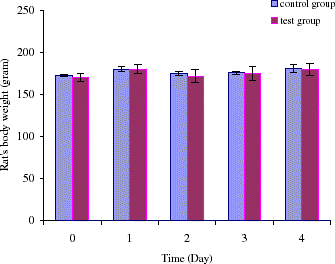
Table 2. p-Value in plasma tyrosine concentration between the control group and the group of oral administration combined with two intravenous injections
Conclusions
The rationale for decreasing systemic tyrosine level to retard melanoma growth is that melanoma requires higher level of tyrosine than normal tissues for growth. To restrict this nutritional situation will slow the growth of the tumor without damaging normal tissues and causing catabolic wasting. We therefore realize that the basis of the management in lowering systemic tyrosine level is to starve the fast growing tumor by limiting tyrosine availability to it without simultaneously producing adverse damages of host tissues.
Previous studies by Lawson and his colleagues indicated that a low level of plasma tyrosine is important to decrease nutrient availability to the melanoma (Lawson et al., Citation[[1985]]). However, in patients, adherence to this tyrosine restricted diet had been very difficult due to fatigue, irritability, hunger, nausea, and abdominal discomfort and severe wasting and weight loss. The tyrosine restriction diet is also a diet very low in protein. We therefore, investigate a novel approach of lowering systemic tyrosine level by combining oral administration of encapsulated tyrosinase and intravenous injection of PolyHb–tyrosinase while a normal diet is continued. Our results have shown that oral administration of encapsulated tyrosinase at three doses a day result in the lowest level of tyrosine among all other doses experiments, but it would take 3 days for this to happen (Yu and Chang, Citation[[2002]]). Intravenous injection of PolyHb–tyrosinase can rapidly decrease tyrosine level in the first hour and maintain a low level for 24 h. In this study, our aim is to combine these two methods to lower systemic tyrosine level. Our results show that systemic tyrosine level can be lowered immediately by the intravenous injection of PolyHb–tyrosinase and keep at this low level by continuing oral administration of artificial cells encapsulated tyrosinase. This combined method does not cause body weight loss in animals, which is a main problem for dietary regimen in restriction of tyrosine and phenylalanine. During our experiments, a normal diet is given and there is no restriction in food intake for animals, which make our experiments easy to apply.
In conclusion, our combined method efficiently decreases systemic tyrosine level without disrupting normal diet intake or causing any weight loss in the animals. This may provide a potential method to inhibit the growth of malignant melanoma. However, further studies need to be carried out on the effects of encapsulated tyrosinase and PolyHb–tyrosinase on lowering tyrosine level in melanoma animal models.
Acknowledgments
TMSC gratefully acknowledges the operating grants from the Medical Research Council of Canada and Canadian Institutes of Health Research. BLY gratefully acknowledges the studentship award from Medical Research Council of Canada and the Canadian Institutes of Health Research.
References
- Abdi E. A., Hanson J., McPherson T. A. Adjuvant chemoimmunotherapy after regional lymphadenectomy for malignant melanoma. Am. J. Clin. Oncol. 1987; 10(2)117–122, [PUBMED], [INFOTRIEVE]
- Bezwoda W. R. The treatment of disseminated malignant melanoma with special reference to the role of interferons, vinca alkaloids and tamoxifen. Cancer Treat. Rev. 1997; 23(1)17–34, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Bourget L., Chang T. M. S. Phenylalanine ammonia-lyase microencapsulated in microcapsules for the depletion of phenylalanine in plasma in phenylketonuric rat model. Biochim. Biophys. Acta 1986; 883: 423–438
- Bourget L., Chang T. M. S. Effects of oral administration of artificial cells microencapsulated phenylalanine ammonia-lyase on intestinal amino acids of phenylketonuric rats. Biomater. Artif. Cells Artif. Organs 1989; 17(2)161–181, [PUBMED], [INFOTRIEVE]
- Chang T. M. S. Monograph on Artificial Cells. Springfield, ILUSA 1972, Charles C. Thomas (free access at http://www.medicine. mcgill. ca/artcell/1972bookCover.pdf)
- Chang T. M. S. Artificial cells with regenerating multienzyme systems. Method. Enzymol. 1985; 112: 195–203
- Chang T. M. S. Monograph on Blood Substitutes: Principles, Methods, Products and Clinical Trials. Karger Landes Systems, Basel, New York 1997, (free access at: http://www.medicine.mcgill.ca/ artcell/bloodsubvoll.pdf)
- Chang T. M. S., Bourget L., Lister C. A new theory of enterorecirculation of amino acids and its use for depleting unwanted amino acids using oral enzyme-artificial cells, as in removing phenylalanine in phenylketonuria. Artif. Cells Blood Substit. Immobil. Biotechnol. 1995; 25: 1–23, [CSA]
- Chang T. M. S., Prakash S. Procedure for microencapsulation of enzymes, cells and genetically engineered microorganisms. Mol. Biotechnol. 2001; 17: 249–260, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Charles M. H. Monograph on Cancer Treatment. W.B. Saunders, PhiladelphiaUSA 2001
- Cocconi G., Bella M., Calabresi F., Tonato M., Canaletti R., Boni C., Buzzi F., Ceci G., Corgna E., Costa P. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. New England J. Med. 1992; 327: 516–523
- Cooper J. S. The evolution of the role of radiation therapy in the management of mucocutaneous malignant melanoma. Hematol. Oncol. Clin. North Am. 1998; 849–862, [INFOTRIEVE]
- Demopoulos H. B. Effects of reducing the phenylalanine-tyrosine intake of patients with advanced malignant melanoma. Cancer 1966; 9: 657–664
- Fraker D. L. Hyperthermic regional perfusion for melanoma and sarcoma of the limbs. Curr. Probl. Surg. 1999; 36: 841–907, [PUBMED], [INFOTRIEVE], [CSA]
- Fronticelli C., Bucci E. Conformation and functional characteristics of bovine hemoglobin. Method Enzymol. 1994; 231: 150–163
- Fronticelli C., Bucci E., Orth C. Solvent regulation of oxygen affinity in hemoglobin. J. Biol. Chem. 1984; 259(17)10841–10844, [PUBMED], [INFOTRIEVE]
- Fronticelli C., Sanna M. T., Perez-Alvarado G. C., Karavitis M., Lu A. L., Brinigar W. S. Allosteric modulation by tertiary structure in mammalian hemoglobins Introduction of the functional characteristics of bovine hemoglobin into human hemoglobin by five amino acid substitutes. J. Biol. Chem. 1995; 270(51)30588–30592, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ge X., Fu Y. M., Li Y. Q., Meadows G. G. Activation of caspases and cleavage of Bid are required for tyrosine and phenylalanine deficiency-induced apoptosis of human A375 melanoma cells. Arch. Biochem. Biophys. 2002; 403: 50–58, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Gennari R., Bartolomei M., Testori A., Zurrida S., Stoldt H. S., Audisio R. A., Geraghty J. G., Paganelli G., Veronesi U. Sentinel node localization in primary melanoma, preoperative dynamic lymphoscintigraphy, intraoperative gamma probe, and vital dye guidance. Surgery 2000; 127: 19–25, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Halvorsen S., Gjessing L. R. Studies on tyrosinosis 1. Effect of low-tyrosine and low-phenylalanine diet. British. Med. J. 1964; 2: 1171–1173
- Lawson D. H., Stockton L. H., Bleier J. C., Acosta P. B., Heymsfield S. B., Nixon D. W. The effect of a phenylalanine and tyrosine restriction diet on elemental balance studies and plasma aminograms of patients with disseminated malignant melanoma. Am. J. Clin. Nutr. 1985; 41: 73–84, [PUBMED], [INFOTRIEVE]
- Meadows G. G., Oeser D. E. Response of B16 melanoma-bearing mice to varying dietary levels of phenylalanine and tyrosine. Nutr. Rep. Int. 1983; 28: 1073–1082
- Meadows G. G., Pierson H. F., Abdallah R. M., Desai P. R. Dietary influence of tyrosine and phenylalanine on the response of B16 melanoma to carbidopa-levodopa methyl ester chemotherapy. Cancer Res. 1982; 42: 3056–3063, [PUBMED], [INFOTRIEVE]
- Meadows G. G., Zhang H., Ge X. Specific amino acid deficiency alters the expression of genes in human melanoma and other tumor cell lines. J. Nutr. 2001; 131: 3047S–3050S, [PUBMED], [INFOTRIEVE]
- Meisenberg B. High-dose chemotherapy and autologous stem cell support for patients with malignant melanoma. Bone Marrow Transplant. 1996; 17: 903–906, [PUBMED], [INFOTRIEVE], [CSA]
- Pavey S., Gabrielli B. Alpha-melanocyte stimulating hormone potentiates p16/CDKN2A expression in human skin after ultraviolet irradiation. Cancer Res. 2002; 62: 875–880, [PUBMED], [INFOTRIEVE]
- Pyrhonen S., Hahka-Kemppinen M., Muhonen T., Nikkanen V., Eskelin S., Summanen P., Tarkkanen A., Kivela T. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer 2002; 95(11)2366–2372, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Quan W. D., Mitchell M. S. Immunology and immunotherapy of melanoma. Cancer Treat. Res. 1993; 65: 257–277, [PUBMED], [INFOTRIEVE]
- Rosenberg S. A., Kawakami Y., Robbins P. F., Wang R. Identification of the genes encoding cancer antigens: implications for cancer immunotherapy. Adv. Cancer Res. 1996; 70: 145–177, [PUBMED], [INFOTRIEVE], [CSA]
- Rosenberg S. A., Lotze M. T., Yang J. C., Topalian S. L., Chang A. E., Schwartzentruber D. J., Aebersold P., Leitman S., Linehan W. M., Seipp C. A. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J. Natl. Cancer Inst. 1993; 85: 622–632, [PUBMED], [INFOTRIEVE]
- Roses D. F., Provet J. A., Harris M. N., Gumport S. L., Dubin N. Prognosis of patients with pathologic stage II cutaneous malignant melanoma. Ann. Surg. 1985; 201: 103–107, [PUBMED], [INFOTRIEVE]
- Serrone L., Zeuli M., Sega F. M., Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J. Exp. Clin. Cancer Res. 2000; 19(1)21–34, [PUBMED], [INFOTRIEVE], [CSA]
- Veronesi U., Cascinelli N. Narrow excision (1-cm margin) A safe procedure for thin cutaneous melanoma. Arch. Surg. 1991; 126: 438–441, [PUBMED], [INFOTRIEVE]
- Waalkes T. P., Udenferiend S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J. Lab. Clin. Med. 1957; 11: 733–736
- Wolff S. N., Herzig R. H., Fay J. W., LeMaistre C. F., Frei-Lahr D., Lowder J., Bolwell B., Giannone L., Herzig G. P. High-dose thiotepa with autologous bone marrow transplantation for metastatic malignant melanoma: results of phase I and II studies of the North American Bone Marrow Transplantation Group. J. Clin. Oncol. 1989; 7: 245–249, [PUBMED], [INFOTRIEVE]
- Wong J. H., Cagle L. A., Morton D. L. Surgical treatment of lymph nodes with metastatic melanoma from unknown primary site. Arch. Surg. 1987; 122: 1380–1383, [PUBMED], [INFOTRIEVE]
- Wong J. H., Steinemann S., Yonehara C., Coel M. N., Ko P. J., Tonaki K., Tauchi P. Sentinel node staging for cutaneous melanoma in a university-affiliated community care setting. Ann. Surg. Oncol. 2000; 7: 450–455, [PUBMED], [INFOTRIEVE], [CSA]
- Yu B., Chang T. M. S. Artificial cells for the removal of systemic tyrosine in rats. Proc. Can. Fed. Biol. Soc. 45th Annu. Meet. (CFBS). 2002; 76
- Yu B., Chang T. M. S. In vitro enzyme kinetics of microencapsulated tyrosinase. Artif. Cells Blood Substit. Immobil. Biotechnol. 2002; 30: 533–546, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Yu B., Chang T. M. S. Effects of long term oral administration of microencapsulated tyrosinase on maintaining decreased systemic tyrosine levels in rats. Pharmaceutical Sciences 2004, (in press)[CSA]
- Yu B., Chang T. M. S. In vitro and in vivo effects of polyhemoglobin-tyrosinase on murine B16F10 melanoma, (submitted)
- Yu W. P., Chang T. M. S. Submicron polymer membrane hemoglobin nanocapsules as potential blood substitutes: preparation and characterization. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996; 24(3)169–183, [PUBMED], [INFOTRIEVE], [CSA]