Abstract
A tissue based biosensor for the determination of phenol was developed by using Jerusalem artichoke (Helianthus tuberosus) in combination with a dissolved oxygen (DO) probe. The tissue electrode response depends linearly on phenol concentration between 0.002 and 0.010 µM in 10 min response time. Maximum electrode response was found in phosphate buffer at pH 8.0 and 35°C. The reproducibility of the enzyme electrode was also tested by using standard phenol solutions (0.005 µM). The standard deviation (SD) and variation coefficient (cv) were calculated as ±1.4 × 10−4 µM and 3.1%, respectively.
Introduction
Phenolic compounds are released into the environment by a large number of industrial wastes. Many of these phenolic compounds have toxic effects on animals and plants, resulting in an acute environmental problem (Haghighi et al., Citation[[2003]]; Jolivalt et al., Citation[[2000]]; Lopez-Avila and Hill, Citation[[1997]]; Marco and Barcelo, Citation[[1996]]; Metzger et al., Citation[[1998]]; Skla’dal et al., Citation[[2002]]). So the monitor and control of these pollutants are greatly important for protection of the environment. For phenol determination various spectrometric and chromatographic methods are in common use. Instead of those conventional methods, biosensor could be a cheap and easy alternative, getting increasing attention in the literature (Campanella et al., Citation[[1993]]; Cosnier and Popescu, Citation[[1996]]; Dennison et al., Citation[[1995]]). Biosensor based on polyphenol oxidase (PPO) may provide a promising method for a simple, fast and sensitive detection of phenolic compounds. But many of them had limited lifetime due to enzyme inactivation by the biocatalytically generated quinine products. Thus, development of good immobilization methods and materials to improve the biosensor stability is very significant (Hall et al., Citation[[1988]]; Kjellen and Neujahr, Citation[[1980]]; Kulys and Schmid, Citation[[1981]]; Mokower et al., Citation[[1996]]; Rogers, Citation[[1995]]). Plant and animal tissues have been successfully employed as biocatalytic components in the construction of biosensors for about two decades. As compared to biosensors with immobilized isolated and pure enzymes, tissue based biosensors show potential advantages of low cost, high stability, longer life time and high level activity. Since the first plant tissue based electrode was reported in 1981, many plant tissue based biosensors have been developed. Most of the plant tissue based biosensors have coupled tissues either to amperometric electrodes, potentiometric elements (transducers) or to a combination of these systems with gas-sensing elements (Baoxin et al., Citation[[2002]]).
In this study, we described a biosensor for the determination of phenol based on Jerusalem artichoke (Helianthus tuberosus) plant tissue. The enzyme Polyphenol oxidase (PPO) in the Jerusalem artichoke has been well characterized in previous works (Zawistowski et al., Citation[[1987]]; Ziyan and Pekyardimci, Citation[[2003]]). Enzymatic reaction due to the action of polyphenol oxidases occurred in the plant tissue can be monitored amperometrically by using oxygenmeter. The enzymatic reaction is as follows:
Plant tissue homogenizate was immobilized directly on DO probe by copolymerization with gelatin, using the bifunctional agent glutaraldehyde as a cross linker (Akyilmaz and Dinckaya, Citation[[2000]]).
Materials and Methods
Materials
225 bloom calf skin gelatin and glutaraldehyde were obtained from Sigma Chem. Co. (St. Louis, MO, USA). All other chemicals were of analytical grade. Jerusalem artichoke were obtained from a local supermarket and stored at 4°C until used.
Apparatus
WTW InoLab Oxi Level 2 model dissolved oxygenmeter based on amperometric mode was used for the experiments. All signals were recorded as dissolved oxygen level (mg/L).
Electrode Preparation
Jerusalem artichoke tissues (0.5 g) were peeled and broken into small pieces and then were homogenized in 0.3 mL of phosphate buffer (0.05 M, pH 7.5). Freshly prepared tissue samples were used for each day.
The tissue homogenate (100 µL) and gelatin (10 mg) were mixed at 38°C in 200 µL of phosphate buffer (pH 7.5, 50 mM). Then, the solution spread over the DO probe membrane and allowed to dry at 4°C for 1 h. Finally, it was immersed in 2.5% glutaraldehyde in 50 mM phosphate buffer (pH 7.5) for 5 min (Ertaş et al., Citation[[2000]]).
Artificial waste water composition; 50 g/L NaCl and 100 g/L phenol in 1 M HCl solution (Livingston, Citation[[1993]]).
Measurement
To determine the phenol concentration, oxygen consumption which was occurred in enzymatic reaction at the immobilized plant tissue membrane covered on top of the dissolved oxygen probe was measured. Measurements were performed by standard curves which were obtained by the detection of consumed oxygen level, related to phenol concentration in the reaction medium. All the measurements were done at 35°C by using a thermostatic reaction cell.
Results and Discussion
Enzyme Electrode Optimization
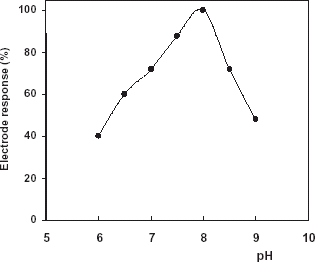
Effect of pH
The effect of pH at the range of 6–9 on the electrode response was studied with phenol. showed that the highest response was observed at pH 8.0 in phosphate buffer (50 mM).
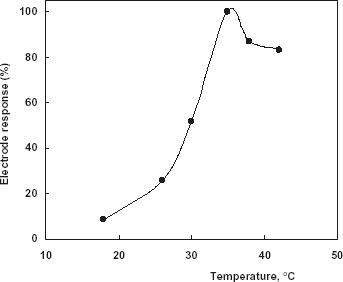
Effect of Temperature
The effect of assay temperature (15–42°C) was examined by using phenol. As is shown in , maximum sensor response was found at 35°C. Therefore, this value was accepted as optimum temperature for all following experiments.
Effect of Tissue Homogenate Amount
Different amounts of tissue (50–150 µL) were used for the biosensor construction and maximum response was obtained with 100 µL of tissue homogenate. At higher amounts, lower responses were observed because of the diffusion problems.
Analytical Characteristics
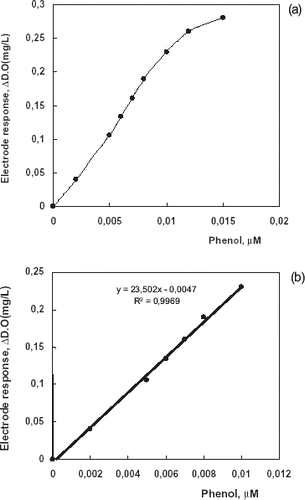
Linear Range
Tissue electrode response depends linearly on phenol concentration between 0.002 and 0.010 µM (a) in 10 min response times in phosphate buffer (pH 8.0). Linear graph (b), defined by the equation y = 23.502 × −0.0047, (R2 = 0.9969) was obtained, for phenol. Linearity was found in the range of 0.002–0.010 µM. At higher concentrations, standard curve showed a deviation from linearity. The deviation was due to insufficient amounts of dissolved oxygen which is a co-substrate of the enzyme.
Reproducibility
The reproducibility of the enzyme electrode was tested by 7 average standard solutions containing equal amounts of phenol (0.005 µM). The standard deviation (SD) and variation coefficient (cv) were calculated as ±1.4 × 10−4 µM and 3.1% (n = 7), respectively.
Thermal Stability
The thermal stability experiments were performed 35°C in working buffer solution. Our data showed that after 6 h no decrease occurred in biosensor response. After that, 20% decrease of the response was observed up to 8th hour. During this time approximately 25 measurements have been made.
Sample Application
The tissue biosensor was applied in waste water sample. For this aim, artificial waste water sample were prepared as described in material and methods. The sample which included known amount of phenol (0.005 and 0.01 µM) was used as stock substrate solutions with different dilutions by working buffer and added to the reaction cell after equilibration and then the change in oxygen amount was measured. Phenol amounts in artificial waste water samples were calculated from calibration curve as 0.0048 ± 0.00017 µM and 0.0095 ± 0.00042 µM, respectively. The signals obtained from the waste samples were found to be very similar with the standard phenol solutions having the same concentration. Results are expressed as mean ± S.D, (n = 4). Our findings showed that, Jerusalem artichoke based tissue biosensor could be a good alternative for the phenol detection without requiring sample pretreatment. Moreover, acidic and salty nature of the sample didn’t interfere to our measurements.
Actually biotechnological processes have been employed in several industrial productions, in biomedical applications and in environmental remediation. Interest was focused on biomediators as purified enzymes or metabolic pathways of cells, tissues etc. with specific biochemical properties for analytical and either production or degradation applications (Rogers, Citation[[1998]]). All our data showed that the obtained biosensors could be used as simple, rapid and direct methods of determining phenol in various media such as waste water samples with different compositions.
References
- Akyilmaz E., Dinckaya E. A mushroom (Agaricus bisporus) tissue homogenate based alcohol oxidase electrode for alcohol determination in serum. Talanta 2000; 53: 505–509, [CSA]
- Baoxin L., Zhujun Z., Yan J. Plant tissue-based chemiluminescence flow biosensor for determination of unbound dopamine in rabbit blood with on-line microdialysis sampling. Biosens. Bioelectron 2002; 17: 585–589, [CROSSREF], [CSA]
- Campanella L., Beone T., Sammartino M. P., Tomassett M. Determination of phenol in wastes and water using an enzyme sensor. Analyst 1993; 118: 979–986, [CROSSREF], [CSA]
- Cosnier S., Popescu I. C. Poly(amphiphilic pyrrole)-tyrosinase-peroxidase electrode for amplified flow injection-amperometric detection of phenol. Anal. Chim. Acta 1996; 319: 145–151, [CROSSREF]
- Dennison M. J., Hall J. M., Turner A. P. F. Gas-phase microbiosensor for monitoring phenol vapour at ppb levels. Anal. Chem. 1995; 67: 3899–3927
- Ertaş N., Timur S., Akyılmaz E., Dinçkaya E. Specific determination of hydrogen peroxide a catalase biosensor based on mercury thin film electrodes. Turk. J. Chem. 2000; 24: 95–99
- Haghighi B., Gorton L., Ruzgas T., Jönsson J. Characterization of graphite electrodes modified with laccase from Trametes versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Anal. Chim. Acta 2003; 224318: 1
- Hall G. F., Best D. J., Turner A. P. F. Amperometric enzyme electrode for the determination of phenols in chloroform. Enzyme Microb. Technol. 1988; 10: 543–546, [CROSSREF], [CSA]
- Jolivalt C., Brenon S., Caminade E., Mougin C., Pontie M. Immobilization of laccase from Trametes versicolor on a modified PVDF microfiltration membrane: characterization of the grafted support and application in removing a phenylurea pesticide in wastewater. J. Membrane Sci. 2000; 180: 103–113, [CROSSREF], [CSA]
- Kjellen K. G., Neujahr H. Y. Enzyme electrode for phenol. Biotechnol. Bioeng. 1980; 22: 299–310, [PUBMED], [INFOTRIEVE], [CSA]
- Kulys J., Schmid R. D. A sensitive enzyme electrode for phenol monitoring. Anal. Lett. 1981; 14: 377–397
- Livingston A. G. A novel membrane reactor for detoxifying industrial waste water: I. Biotech. Bioeng. 1993; 41: 915–922, [CSA]
- Lopez-Avila V., Hill H. H. Field analytical chemistry. Anal. Chem. 1997; 69: 289–305, [CROSSREF]
- Marco M. P., Barcelo D. Environment applications of analytical biosensors. Measurement Sci. Technol. 1996; 7: 1547–1572, [CROSSREF], [CSA]
- Metzger J., Reiss M., Hartmeier W. Amperometric phenol biosensor based on a thermostable phenol hydroxylase. Biosens. Bioelectron 1998; 13: 1077–1082, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mokower A., Eremenko A. V., Streffer K., Wollenberger U., Scheller F. W. Tyrosinase-glucose dehydroenase substrate-recycling biosensor: a highly sensitive measurement of phenolic compounds. J. Chem. Tech. Biotechnol. 1996; 65: 39–44, [CROSSREF], [CSA]
- Rogers K. R. Biosensors for environmental applications. Biosens. Bioelectron 1995; 10: 533–541, [CROSSREF], [CSA]
- Rogers K. R. Biosensor technology for environment measurement. Encyclopedia of Environmental Analysis and Remediation, R. A. Meyers. John Wiley and Sons, New York 1998; pp. 755–768
- Skla’dal P., Mozorova N. O., Reshetilov A. N. Amperometric biosensors for detection of phenol using chemically modified electrodes containing immobilized bacteria. Biosens. Bioelectron 2002; 17: 867–863, [CROSSREF], [CSA]
- Zawistowski J., Weselake R. J., Blank G., Murray E. D. Fractionation of Jerusalem artichoke phenolase by immobilized copper affinity chromatography. Phytochemistry 1987; 26: 2905–2907, [CROSSREF], [CSA]
- Ziyan E., Pekyardimci S. Characterization of polyphenol oxidase from Jerusalem artichoke (Helianthus tuberosus). Turk J. Chem. 2003; 27: 217–225, [CSA]