Abstract
Over 100 preclinical studies in several small and large animal species were performed to evaluate the safety and efficacy of diaspirin crosslinked hemoglobin (DCLHb; Baxter Healthcare Corp.) as an oxygen therapeutic. During the preclinical evaluation of DCLHb, myocardial lesions were observed following the administration of DCLHb to certain species. These lesions were characterized as minimal to moderate, focal-to-multifocal myocardial degeneration and/or necrosis that were scored using a severity scale of minimal to marked in relative severity. The lesions were typically observed 24–48 h after single topload infusions of DCLHb into rhesus monkeys or pigs at doses as low as 200 or 700 mg/kg, respectively. Dogs, sheep, and rats did not develop these lesions after single-dose administrations of DCLHb. The left ventricular myocardium, typically near the base of or including the papillary muscles, was the most severely affected region, followed by the intraventricular septum and the right ventricle. The left and right atria were usually not affected. In a study in rhesus monkeys, morphometric analysis revealed that these lesions comprised less than 3% of the total myocardium. Although increases in serum enzyme activities (AST, CK, LDH) were observed after infusion of DCLHb, myocardial-related isoenzymes did not increase. ECG analysis and echocardiography were not altered by these lesions, and there was no observable adverse effect on myocardial function. Polymerization of DCLHb reduced, but did not eliminate, the incidence and severity of the lesions. However, infusion of hemoglobin solutions with reduced reaction rates with nitric oxide (NO) resulted in a significant decrease in lesion incidence and severity, while administration of L-NAME, an NO synthase inhibitor, resulted in the appearance of lesions that were indistinguishable from those induced by hemoglobin, suggesting that reduction in normal NO levels is an important mechanistic factor. Overall, the presence of myocardial lesions represents a histopathologic finding that must be considered during the preclinical testing and development of new HBOCs.
Introduction
The quest by medical researchers for a safe intravenous acellular oxygen-carrying solution has continued for over 60 years (Amberson, Citation[[1937]]). This search has been driven by multiple factors, including the risk posed by blood-borne pathogens, the necessity to match the blood type of the donor with the recipient, the short shelf-life of stored blood, and the immunosuppressive effects that may follow blood transfusion (Linden and Bianco, Citation[[2001]]). As a result of these concerns, several modified hemoglobin solutions are currently being developed as oxygen-carrying resuscitation solutions.
Diaspirin crosslinked hemoglobin (DCLHb, Baxter Healthcare Corp.) is a modified human hemoglobin solution produced by reacting deoxygenated human hemoglobin with the crosslinking agent, bis(3,5-dibromosalicyl) fumarate (DBBF), to form a stabilized tetramer that is covalently linked between the alpha globin chains (Chatterjee et al., Citation[[1986]]; Synder et al., 1987; Walder et al., Citation[[1979]]; Zaugg et al., Citation[[1980]]). During the initial toxicological evaluations of DCLHb, single dose studies were performed in several standard animal species. In studies in rats and dogs, no adverse effects of DCLHb on the heart were observed with doses up to 40 mL/kg (4000 mg DCLHb/kg). Dose escalation and repeat dosing studies with DCLHb were subsequently conducted in cynomolgus monkeys. During the microscopic examination of heart tissues in these studies, myocardial degeneration and/or necrosis of mild-to-moderate severity was observed in animals treated with moderate to high doses of DCLHb.
After discovery of these lesions, a variety of different experiments were performed to better understand their etiology, pathogenesis, and clinical significance. The initial objective of this work was to develop a relevant, sensitive, and reproducible animal screening model in which heart lesions similar to those seen in primates could be produced in response to hemoglobin administration. Another objective was to fully characterize the myocardial lesion in those species in which this pathology was observed. Finally, the mechanism of lesion development was studied and interactions designed to mitigate lesion development were assessed. The purpose of this review is to summarize the results obtained from this body of work.
Overview
Animal Model Development
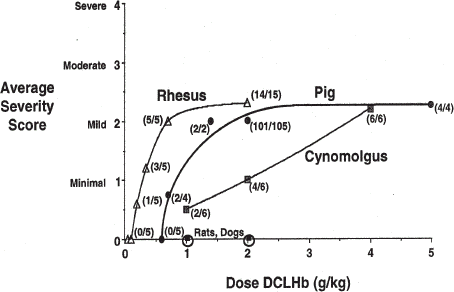
Although originally found in cynomolgous monkeys, and subsequently observed in African green monkeys, both of these species were significantly less sensitive than rhesus monkeys to the development of myocardial lesions (). Cynomolgus monkeys infused with 2000 mg/kg of DCLHb typically developed lesions of myocardial degeneration and/or necrosis graded as minimal in severity (1.0 on a scale of 0–4) with an incidence of 67%. In contrast, rhesus monkeys developed more severe heart lesions at relatively low doses of DCLHb.
Figure 1. Dose response characteristics of heart lesion development in primates 48 h after a single infusion of DCLHb.
While these data demonstrate that primates are very sensitive to the development of this lesion, the use of primates for screening purposes was not practical. Therefore, experiments were performed to identify a more cost effective model that would allow rapid and reproducible screening for identifying potential mechanisms and/or co-medicaments with the least number of animal use issues. This goal was challenging since heart lesions had not been observed in experiments performed with dogs, sheep, or rats following single infusions of DCLHb, implying that the no-effects doses in these species were greater than 4000 mg/kg.
Heart lesions with a similar appearance could be produced in rabbits, however 10-fold higher doses of DCLHb (>3000 mg/kg) were required to produce a comparable incidence and severity to that seen in rhesus monkeys. Moreover, in rabbits there was a significant background incidence of degenerative, necrotic, and inflammatory heart lesions in untreated, sham-treated, and control rabbits. The DCLHb induced heart lesions in rabbits also typically had a more pronounced inflammatory component (myocarditis) consisting of interstitial infiltrations of heterophil leukocytes and mononuclear cells resembling lymphocytes and macrophages. Inflammation was sometimes present in the absence of discernible myofiber degeneration or necrosis. Due to these differences, development of this animal model was not continued.
On the other hand, it was found that swine were a very good model because they consistently developed heart lesions after infusion of moderate doses of hemoglobin solutions with a low level of background incidence. In addition, swine are generally recognized as a good species for studying the effects of agents on the cardiovascular system and are also a species that reproduces the hemodynamic responses observed in humans after the infusion of DCLHb with respect to increases in mean arterial blood pressure. Therefore, it was decided to further develop the swine model for cardiac lesion development. With a no-effects dose less than 700 mg/kg, swine appeared to be more sensitive than cynomolgus monkeys and almost as sensitive as rhesus monkeys ().
To ensure comparability between experiments, the swine model used for evaluation of heart lesions was standardized. All experiments utilized young crossbred domestic swine that weighed between 10 and 20 kg. Approximately 24 h prior to dosing animals were anaesthetized and chronic catheters were placed in the jugular vein, and sometimes in the carotid or femoral artery, for infusion of test and control articles and blood sampling for clinical chemistry analysis. Test or control solutions were infused intravenously using an infusion pump at a constant rate of 1 mL/kg/min. When assessing the effect of interventions, a standardized DCLHb dose of 2000 mg/kg was typically infused. This dose was found to be the best compromise between minimizing volume load and consistently producing lesions. As a treated control, human serum albumin (HSA) that was oncotically matched to each test article was infused into separate swine at the same rate and volume. Blood samples were routinely taken at baseline, immediately postinfusion, and at 8, 24, and 48 h postinfusion of test or control article for the measurement of various enzyme levels. Clinical observations were performed throughout the experiments. At approximately 48 h postdosing, the animals were euthanized, a complete necropsy examination was performed, and various tissues, including the heart, were taken for histopathologic evaluation. The heart specimens routinely examined consisted of the atria, left and right ventricular free wall, and inter-ventricular septum, including associated papillary muscle. As noted in more detail below, 48 h postdosing was determined to be the optimal time for sacrifice because this interval allowed enough time for the lesion to develop, but yet was not so long after the initial insult that damaged myocardial cells might already be removed and cleared by repair processes in the body.
Lesion Characterization
Once sensitive and reproducible animal models were identified, the hemoglobin-induced myocardial lesions were characterized in more detail. In primates, myocardial lesions observed after hemoglobin infusion consist of a minimal to moderate myocardial degeneration characterized by cytoplasmic swelling and vacuolization of myofibers, occurring primarily in the left ventricle and/or septum. The lesions are usually focal or multifocal in distribution, sometimes only involving a few cells, although occasionally they may be locally extensive. Often the degeneration is associated with foci of coagulative myofiber necrosis that display a homogeneous to granular eosinophilic staining cytoplasm. Enlargement of the nuclei (karyomegaly) of myocytes and minimal to mild interstitial fibrosis are also frequently associated with the degenerative lesions. The karyomegaly is interpreted as a reactive change. In some animals, a mild lymphocytic inflammatory infiltrate is also present.
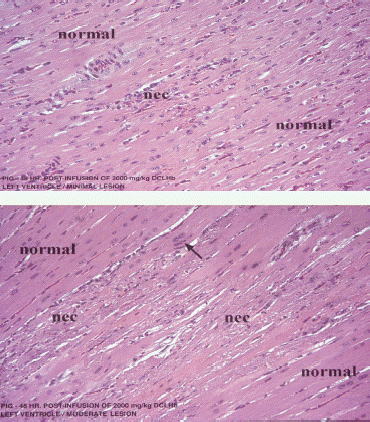
Similar to the lesions observed in primates, the lesions found in swine were described as myocardial degeneration and/or necrosis, with a focal to multifocal distribution, and of minimal to moderate severity. The myocardial degeneration was characterized by focal cytoplasmic swelling and slight hypereosinophilia of myofibers, whereas necrosis was evident as areas of moderate to marked homogeneous to granular eosinophilic cytoplasmic staining with shrinkage (pyknosis), fragmentation (karyorrhexis) or lysis (karyolysis) of the nuclei. Necrotic areas were usually associated with a mononuclear inflammatory infiltrate consisting of macrophages and a lesser number of lymphocytes. Mineralization of cellular debris could also be observed occasionally at some sites of necrosis. Shown in are microscopic changes involving the left ventricle of swine illustrating typical lesions of necrosis classified as minimal or moderate following a single IV infusion of 2000 mg/kg DCLHb.
Figure 3. Photomicrographs of H&E stained sections of myocardium from the left ventricle of different swine following infusion of 2000 mg/kg of DCLHb, illustrating typical heart lesions of different severity. (nec = necrosis).
To quantify the characteristics of the cardiac lesions using anatomic pathology, both incidence and severity parameters were utilized. Incidence was defined as the number of hearts that exhibited any evidence of lesion formation divided by the total number of hearts examined (e.g., 2/4). Severity was a measure of lesion intensity and extent that was scored by the evaluating pathologist on an ascending scale of 0–4. Lesions of Grade 1 were considered minimal, Grade 2 were considered mild, Grade 3 were moderate, and Grade 4 lesions were severe. In a given group of tissue specimens, an overall average severity score was calculated by summing the severity grades for each affected heart and dividing by the total number of hearts evaluated in that group.
By combining data from several studies, the variation of lesion incidence and severity after the administration of single doses of DCLHb was defined in rhesus monkeys (). Above the no-effect level of 100 mg/kg, a dose-response relationship was observed, with a 100% incidence and maximization of the average lesion severity at a score of approximately 2.3 at 700 mg/kg. At substantially higher doses, no significant increase in the severity of the lesion was observed. To examine the severity of the lesions quantitatively, a morphometry study was conducted in rhesus monkeys after the infusion of 2000 mg/kg of DCLHb. In this study, five animals were infused intravenously with 20 mL/kg of a 10 g% DCLHb solution at a rate of 1.0 mL/kg/min, the animals were sacrificed, seven days postinfusion, and the heart tissue collected, fixed, sectioned, and examined by morphometric analysis. Myocardial lesions were observed in four out of five monkeys. In the affected hearts, the mean fraction of tissue with degeneration or necrosis was 1.26% (range = 0.15–2.92%) (). The most sensitive tissue was the left ventricular papillary muscle, followed by the left ventricular free wall and inter-ventricular septum. The right ventricle was sometimes affected, but to a much lesser degree. The atria were almost never affected.
Figure 4. Percent of heart tissue with degeneration and/or necrosis 7 days following infusion of 2000 mg/kg of DCLHb into rhesus monkeys.
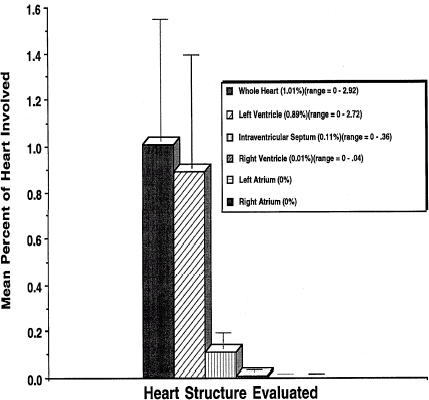
Table 1. Incidence and severity of heart lesions in primates
In this experiment, as well as in many subsequent experiments in a number of different animal species, an attempt was made to identify a clinical pathology marker for myocardial injury that would allow for more rapid and sequential monitoring of lesion development. Unfortunately, to date no statistically significant increases in typical markers of myocardial injury, such as the myocardial isoenzyme of creatine kinase (CK-MB) or the lactic dehydrogenase isoenzyme LDH-1, were observed following infusion of large and repeated doses of DCLHb, even into sensitive species such as the rhesus monkey. These studies were evaluated by an outside expert investigator that utilized specific monkey immunoassays for analysis of CK-MB activity. The small percentage of myocardium involved in the monkeys is consistent with the observation that no significant levels of cardiac specific enzymes were identified in the plasma after hemoglobin infusion. In fact, to date no surrogate marker of myocardial injury has been identified.
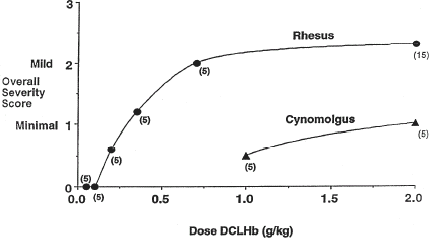
The “self-limiting” nature of the severity of the lesion is also apparent in the data accumulated in a 28-day repeat dose toxicity study in rhesus monkeys. This study investigated the toxicity of DCLHb in awake rhesus monkeys following daily infusions of DCLHb for 28 days, with doses ranging from 1000 to 4000 mg/kg/day. The planned sacrifice intervals were either Day 29 (after receiving 28 doses) or Day 64, with the latter providing 28 days of daily dosing followed by a recovery period. Although some animals received cumulative doses as large as 112,000 mg/kg of DCLHb, the severity of the lesions () was no greater than that seen in the earlier single dose studies. Even more intriguing was the observation that many animals had no histologic evidence of myocardial lesions following infusion of DCLHb at cumulative doses substantially greater than the 100 mg/kg no-effects dose in single dose studies. For example, only three of seven monkeys dosed with 2000 mg DCLHb per kg and examined at Day 29 had histological evidence of myocardial lesions, despite the fact that they each received a cumulative dose of 56,000 mg/kg of hemoglobin. This is in direct contrast to the results in monkeys that received a single 2000 mg/kg infusion (), in which the incidence of heart lesions was 14/15 monkeys. The explanation for this difference in response is not known with certainty, but may reflect the competency of the cardiac tissue repair process.
Table 2. Incidence of myocardial degeneration vs. Dose in rhesus monkeys during a 28 day repeat dose study
An advantage of utilizing swine in the study of cardiac lesions was the ability to perform chronic experiments in unanaesthetized animals, which allowed for easier and more thorough examination of cardiovascular function. This permitted assessment of the functional consequences of cardiac lesion development utilizing electrocardiography (ECG). To do so objectively, cardiac function in swine infused with DCLHb or HSA control solutions was compared. DCLHb (2000 mg/kg) or an oncotically matched HSA solution was infused into swine at a rate of 1 mL/kg/min. Cardiac function was assessed preinfusion, and 24 and 48 h postinfusion, by ECG analysis performed by a veterinary cardiologist who was blinded to treatment. The cardiac index and selected clinical chemistry parameters were also measured. At 48 h postinfusion, cardiac tissue was evaluated microscopically. Heart lesions were observed in all six DCLHb treated pigs with an overall pathology score of 2.7, while no lesions were observed in animals infused with HSA. In DCLHb treated pigs, serum aspartate transaminase (AST) concentrations increased from a baseline of 28 ± 2 to 126 ± 8 IU/mL at 48 h postinfusion, and total serum creatine kinase (CK) concentrations increased from a baseline of 20 ± 2 to 67 ± 5 SU/mL. These increases were typical and representative of the response seen following infusion of DCLHb into swine. Yet, none of the animals exhibited disturbances in cardiac rhythm or conduction, although minor changes in T-wave morphology and polarity were observed in both groups. No clinically significant effect on cardiac function by DCLHb could be discerned in this study.
To assess the time course of lesion development, tissues collected from animals sacrificed at different time intervals were examined microscopically. From these examinations, it was concluded that the degenerative myocardial changes appeared as early as 1–6 h postinfusion. Electron microscopy was required to detect the changes at early time points. While some degenerative cells became necrotic, others apparently recovered their normal appearance and function. Necrotic tissue was ultimately removed and subsequently replaced, in part, by fibrous connective tissue. Another component of the recovery process was the enlargement of myocytes adjacent to affected areas, which probably represented a physiologic hypertrophy caused by increased functional demand on the unaffected cells. Morphologic evidence of muscle fiber regeneration was also evident in swine. The long-term consequence of myocardial lesion development was the loss (necrosis) of a small fraction of the myocytes originally present that were replaced by proliferation of connective tissue and possible regeneration of muscle cells.
Co-medicament Experiments
Using the standardized swine model, the mechanism of hemoglobin-induced heart lesion formation and possible methods for mitigation of this process were extensively examined. In considering these experiments, it should be noted that the standardized DCLHb dose of 2000 mg/kg produced heart lesions in 96% of the treated animals with an average severity score of approximately 2 (n = 105, ). Occasionally, lesions were seen in animals that were infused with HSA, but the incidence and severity was extremely low. Background lesions were not routinely seen in normal, untreated swine. For the purposes of this manuscript, experimental results will be summarized.
Table 3. Heart lesions following infusion of DCLHb (reference range in swine)
One set of experiments was designed to assess whether there was a specific contaminant in DCLHb that was responsible for the cardiac pathology (). Infusion of DCLHb that was subjected to an additional chromatographic purification step produced the same results as the standard DCLHb solution, suggesting that contamination was not responsible for causing the lesion. Additionally, infusion of purified, uncross-linked, human stroma-free hemoglobin (SFHb) produced the same heart lesion as DCLHb with the same incidence, albeit with a slightly reduced severity. The reduced severity was probably due to the considerably shorter circulating half-life of unmodified hemoglobin compared to DCLHb due to the rapid excretion of the SFHb through the kidney. This would be expected to somewhat reduce the direct exposure of the heart to the SFHb.
Table 4. Incidence and severity of heart lesions in pigs infused with DCLHb and co-medicaments
Furthermore, infusion of purified swine SFHb into pigs caused the same heart lesion as that seen following infusion of DCLHb or human SFHb, suggesting that this phenomenon is probably a more general property of acellular hemoglobins. These experiments also demonstrated that myocardial lesions were not due to infusion of a human protein into a nonhuman species. Finally, in order to investigate if heart lesion formation could be related to the reduced heme component of DCLHb, the effect of conversion of the heme to the cyanomet form was examined. It was found that conversion to the cyanomet form had no significant effect on the incidence and/or severity of the heart lesion, although this result may have been compromised by in vivo conversion of cyanometHb to reduced Hb.
To gain further insight into the potential mechanism of heart lesion formation, as well as to identify potential interventions that would be clinically useful, the effect of co-administration of many different agents with varying pharmacologic actions was assessed. In addition, the impact of variations in the hemoglobin administration protocol were evaluated. In the typical experiment, the standardized swine testing protocol was utilized with the key independent variable being the comedicament or specific protocol variation. In some cases, a variety of dosing regimens or administration protocols were evaluated with each agent. The primary endpoint in each case was histologic evaluation of the hearts as quantified by the myocardial lesion incidence and overall severity score. Comedicaments and protocol variables that were examined include the following:
Antihypertensives: Nicardipine, adenosine, phenoxybenzamine, propranolol, verapamil, captopril, ATP-MgCl2, metroprolol, halothane, sodium nitroprusside, l-arginine.
Anticoagulants: Aspirin, dipyridamole, heparin.
Anti-inflammatory: Dexamethasone, ibuprofen, benadryl.
Antioxidants: Taurine, vitamin E, selenium, ascorbate, OTC (l-2-oxothizolidine-4-carboxylic acid), MPG (N-2-mercaptopropionyl glycine), oxypurinol, mannitol, lactobionate, carnitine, allopurinol, lipoic acid.
Iron binding: Deferoxamine.
Protocol variations: Topload (hypervolemic) infusion; differing levels of isovolemic exchange transfusion; predosing with hemoglobin; dosing of hemoglobin in hemorrhage/resuscitation protocols; animal source; animal gender; effect of splenectomy, hydration state, or anesthesia; effect of catecholamine depletion before hemoglobin administration.
After extensive testing, no effective comedicament was identified, nor was any definitive mechanism of action elucidated in this series of experiments. Likewise, the administration protocol did not seem critical, as similar lesions were observed if the hemoglobin was administered as a volume load, by exchange/transfusion, or to hemorrhaged animals.
Effects of Hemoglobin Modification
Polymerization
To assess the potential effect of the molecular size of the hemoglobin molecule on the generation of heart lesions, several experiments were performed with different DCLHb derivatives. In one study, DCLHb was treated with gluteraldehyde to create a polydisperse family of hemoglobin polymers. This solution was then diafiltered against a membrane having a nominal 300,000 Dalton molecular weight cut-off. The resulting retentate solution was essentially free of unpolymerized hemoglobin tetramers, while the filtrate was enriched in this molecular weight fraction. After diafiltration into the same electrolyte vehicle, these two solutions were infused into swine. The lesion incidence and overall severity scores were lower in animals that received the polymerized DCLHb retentate (2/5 and 0.5, respectively) compared to those animals treated with filtrate (5/5, 2.6). Similar results were obtained when DCLHb was polymerized with bifunctional polyethylene glycol based reagents. In most cases, both the incidence and severity of the heart lesions could be reduced, but not completely eliminated, by increasing the molecular size of the DCLHb. These data imply that the size of the hemoglobin molecule does have an influence on the generation of heart lesions, but that the lesions could not be completely eliminated in sensitive species simply by polymerization.
Genetic Modifications to Inhibit Hemoglobin Reaction with Nitric Oxide
As part of the investigation into possible mechanisms of cardiac lesion development, the potential role of nitric oxide was investigated. Native hemoglobin interacts very strongly with nitric oxide (NO), a ubiquitous and potent chemical messenger found throughout the body. In vivo, nitric oxide scavenging by hemoglobin occurs primarily via two rapid reactions: the oxidative reaction of NO with oxyhemoglobin to produce nitrate and methemoglobin, and NO binding to deoxyhemoglobin to form a stable complex (Patel, Citation[[2000]]). Both reactions likely contribute to in vivo NO scavenging, with the relative significance depending on local abundances of oxy- and deoxyhemoglobin. There is also evidence that this scavenging of NO may be associated with some of the adverse outcomes observed with the first generation hemoglobins. For example, studies in rats have clearly demonstrated that increases in mean arterial pressure observed immediately after hemoglobin infusion correlate directly with the rate of NO scavenging; as the NO scavenging is decreased, the pressor response is decreased (Doherty et al., Citation[[1988]]). More recently, it has been reported that the chronic inhibition of nitric oxide production by L-NAME causes myocardial infarction in rats (Moreno et al., Citation[[1997]]; Ono et al., Citation[[1999]]).
L-NAME is an inhibitor of the enzyme nitric oxide synthase that produces NO. Infusion of L-NAME into swine resulted in heart lesions similar in incidence, severity, and appearance to the lesions observed after infusion of DCLHb ().
Table 5. Incidence and severity of heart lesions in pigs infused with DCLHb and various co-medicaments
To systematically investigate the role of hemoglobin/NO interactions, a series of genetically altered hemoglobins were produced using recombinant technology. These hemoglobins were specifically designed to exhibit varying rates of reaction with NO. Recombinant hemoglobins with NO scavenging properties similar to those of native human hemoglobin (e.g., rHb1.1 produced by Somatogen) produced heart lesions with the same incidence and severity as those seen with DCLHb. In contrast, recombinantly produced hemoglobin solutions that contained heme-pocket modifications that reduced the rate of nitric oxide interaction exhibited a reduced rate of heart lesion formation after infusion into swine (). More specifically, a hemoglobin variant with a 25-fold decrease in nitric oxide reactivity produced no detectable heart lesions in swine. This variant was internally crosslinked by recombinant techniques, and was very similar to rHb1.1 or DCLHb with respect to molecular weight, oxygen affinity, and oxygen binding kinetics.
Table 6. Incidence and severity of heart lesions in pigs infused with various hemoglobin solutions
As a result of these promising results in swine, this same hemoglobin variant was subsequently tested in rhesus monkeys. In contrast to the results in swine, myocardial lesions were observed in all of the test animals following infusion into monkeys, although the lesion severity was substantially reduced. This led to exploration of the effect of the combination of polymerization and a reduced rate of NO interaction on heart lesion development. To do so, an intramolecularly cross-linked hemoglobin with reduced NO reactivity was polymerized and derivatized with a bifunctional polyethylene glycol reagent. This new material, designated as rHb2.0 for Injection, was evaluated in both single dose and repeat dose studies in rhesus monkeys, as well as in swine and rats. In a single dose toxicity study in rhesus monkeys, no cardiac lesions were observed in animals that were sacrificed 48 h after receiving a single dose of either 500 (n = 8), 1000 (n = 8), or 2000 mg/kg (n = 8) of rHb2.0. In a separate group of monkeys that were sacrificed two weeks after dosing, there was also no evidence of myocardial lesions. In a repeat dose study in which rhesus monkeys received every other day infusions of either 1000 or 2000 mg/kg of rHb2.0 for Injection (10 animals per dose group) for a total of seven infusions over 13 days, only one animal in the high dose group exhibited a myocardial lesion, and it was focal and of minimal severity. Moreover, according to the reviewing pathologist, this lesion was of uncertain association with study drug administration since a background lesion of similar appearance is sometimes observed in monkeys. None of the other monkeys examined at the 48 h sacrifice interval, or in the recovery group sacrificed 14 days after receiving the seventh dose, had any evidence of myocardial lesions. In total, only one of 56 monkeys receiving rHb2.0 for Injection exhibited any finding of myofiber degeneration or necrosis. These data suggest a major role for nitric oxide depletion in the mechanism of myocardial lesion development.
In a swine cardiovascular function/safety study in which the hearts were examined histologically, there were no cardiac lesions observed in swine infused with 2000 mg/kg of rHb2.0 for Injection. However, animals receiving DCLHb as a positive control exhibited cardiac lesions with a similar incidence and severity to those observed in previous studies.
In contrast to first generation hemoglobin solutions, rHb2.0 for Injection did produce observable cardiac changes in rats in a single dose rat toxicity study. However, both the incidence and severity of these lesions did not appear to follow a dose-response relationship and none of the rats in the recovery group, sacrificed 14 days after dosing (n = 10/group), had evidence of myocardial lesions. Interestingly, myocardial lesions attributable to rHb2.0 were not observed in a hemorrhage/resuscitation study performed in rats nor in a separate diabetic rat study. The difference and significance of these findings in rats between the first and second generation HBOCs is not known, although previous testing would suggest that the results in swine and primates are more relevant to man.
Discussion
Over the past decade there has been a substantial effort by Baxter researchers to understand the mechanism of heart lesion formation following the intravenous infusion of hemoglobin solutions (Burhop and Estep, Citation[[2001]]). Characterization of the lesion suggests that there is significant variation among species with respect to susceptibility to the development of this pathology, with swine and primates being the most susceptible, and dogs and rodents being relatively insensitive. In addition, only a small percentage of heart muscle cells appears to be affected in even the most sensitive species, since the fraction of necrotic cells plateaus at moderate dose levels and does not increase even when hemoglobin doses many-fold greater are administered. Another important observation is the fact that lesions are detectable by light microscopy from approximately 24–72 h after hemoglobin administration. Thus, evaluation of tissues at earlier or later time intervals may not detect the presence of this pathology. It is also noteworthy that the development of cardiac lesions is not associated with an elevation of cardiac specific isoenzymes, although some plasma enzyme levels are increased by several-fold after hemoglobin administration.
With respect to mechanism, lesion development appears to be due to the hemoglobin molecule per se, rather than a contaminant, since additional purification of DCLHb did not reduce the incidence or severity of the cardiac pathology. Moreover, the fact that heart lesion development was observed after the administration of either human or swine stroma-free hemoglobin, or a recombinant hemoglobin produced by bacterial fermentation, strongly argues that the finding is attributable to hemoglobin rather than a contaminant, since the contaminant profiles of these preparations is expected to be very different. Variations in hemoglobin administration protocols also did not appear to influence the incidence or severity of heart lesions, suggesting that the phenomenon not due to volume overloading.
To explore other potential mechanisms, efforts were directed to the testing of a large number of compounds whose mechanism of action might be hypothesized to interfere with a putative mechanistic pathway. While numerous agents were evaluated as part of this effort, these studies did not lead to the identification of a definitive mechanism of action.
Several different experts consulted on this issue noted that the myocardial changes observed after hemoglobin infusion are very similar in appearance to those seen after the administration of high doses of sympathomimetic amines such as epinephrine, norepinephrine, and dopamine (Haft, Citation[[1974]]). For example, infusion of dopamine into dogs during a 14 day subacute toxicity study resulted in the formation of myocardial lesions (FDA, Citation[[1973]]). However, the fact that catecholamine depletion did not mitigate the formation of heart lesions in swine after subsequent hemoglobin infusion suggests that this pathway is not involved in the hemoglobin induced response. Several experts have also noted that the lesions appear different in their histological appearance, time-course, and severity than those seen after infusion of other cardiotoxic drugs, such as Adriamycin (Buja et al., Citation[[1973]], Citation[[1974a]], Citation[b]).
On the other hand, modification of the structure of the hemoglobin molecule in two distinct manners has been shown to mitigate lesion development. Both polymerization to create hemoglobin polymers with an average molecular weight of several hundred thousand Daltons, and mutation of heme pocket amino acids to reduce the rate of heme interaction with nitric oxide, result in a reduction in the incidence and/or severity of cardiac lesion formation. It is believed that polymerization acts to reduce the rate of hemoglobin extravasation into heart tissue and thereby lowers the hemoglobin concentration near sensitive cells, while modification to reduce the rate of interaction with nitric oxide results in a reduced rate of NO scavenging which has a salutory effect on lesion development. Moreover, these two modifications appear to be at least somewhat additive in that the lowest incidence of heart lesion development in rhesus was achieved with hemoglobin molecules that were both polymerized and altered to reduce the inherent rate of NO scavenging. On the basis of these observations, an hypothesis can be generated for the mechanism by which hemoglobin induces the formation of cardiac lesions. The pertinent facts are that:
Hemoglobin scavenges nitric oxide.
Infusion of nitric oxide inhibitors can produce myocardial lesions.
The papillary muscle of the left ventricle is the most sensitive myocardial tissue with respect to the adverse effects of hemoglobin infusion and nitric oxide inhibition.
The left ventricular papillary muscle is one of the highest oxygen consuming tissues in the body.
Infusion of hemoglobin into sensitive species, such as pigs and monkeys, produces significant increases in blood pressure and thereby an increase in after-load on the heart. This results in increasing myocardial oxygen demand that can result in a localized tissue hypoxia.
Polymerization of hemoglobin, which can slow down, but not completely eliminate, extravasation of hemoglobin from the vascular space, reduces both the severity and incidence of the myocardial lesions.
Recent data suggest that inhibition of nitric oxide synthesis increases mitochondrial oxygen consumption and may also affect Ca++ hemostasis (Arstall and Kelly, Citation[[1999]]; Bernstein et al., Citation[[1996]]; Boveris et al., Citation[[2000]]; Henry and Guissani, Citation[[1999]]; Shen et al., Citation[[1994]]; Zhao et al., Citation[[1999]]).
When considered as a whole, these facts suggest that infusion of hemoglobin leads to enhanced oxygen consumption throughout the body as a consequence of a reduction in tissue levels of nitric oxide. In the heart, especially in the papillary muscle, there is an increase in mitochondrial oxygen consumption due not only to the decrease in NO levels, but also as a consequence of the increased after-load due to peripheral vasoconstriction. As a result, oxygen demand may exceed oxygen supply in the most sensitive cells in the heart, leading to microscopic areas of hypoxia, cell injury, and ultimately death. Likewise, as a result of interactions between hemoglobin and NO, there may be alterations in calcium hemostasis that may ultimately lead to myocardial cell degeneration and necrosis (i.e., produce contraction band necrosis). The inflammatory response that is seen in conjunction with the necrosis is likely a secondary event that represents removal, by macrophages, of necrotic myocardial cells.
It is important to note that to date no evidence of a hemoglobin-induced myocardial lesion has been observed in man. Furthermore, there have been no increases seen in enzymatic markers of myocardial injury such as CK-MB or troponin-I in any of the human clinical trials conducted with DCLHb. However, the detection of hemoglobin induced heart lesions in humans is confounded by the fact that patients treated with hemoglobin therapeutics have myocardial damage from other causes. It is therefore unclear whether the lesions observed in swine or primates occur in man. Nevertheless, the presence of myocardial lesions represents a histopathologic finding that must be considered during the testing and development of new hemoglobin therapeutics and confirmation of the basic mechanism of lesion development would be helpful in estimating the potential clinical relevance of this finding.
Acknowledgments
The authors would like to thank the host of technical staff across a variety of research and development groups at Baxter who made significant contributions to this research. Without all of their help, this work would not have been possible. The expert advice and scientific input of outside consultants such as Dr. Robert Jennings from Duke University is also greatly appreciated.
References
- Amberson W. R. Substitutes for blood. Biol. Rev. 1937; 12: 48–86
- Arstall M. S., Kelly R. A. The role of nitric oxide in heart failure. Coronary Artery Dis. 1999; 10(5)301–308
- Bernstein R. D., Ochoa F. I., Xu X., Forfia P., Shen W., Thompson C. I., Hintze T. H. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ. Res. 1996; 79: 840–848, [PUBMED], [INFOTRIEVE]
- Boveris A., Costa L. E., Poderoso J. J., Carreras M. C., Cadenas E. Regulation of mitochondrial respiration by oxygen and nitric oxide. NY Academy of Sci. 2000; 899: 121–135
- Buja L. M., Ferrans V. J., Mayer R. J., Roberts W. C., Henderson E. S. Cardiac ultrastructural changes induced by daunorubicin therapy. Cancer 1973; 32(4)771–788, [PUBMED], [INFOTRIEVE]
- Buja L. M., Ferrans V. J., Roberts W. C. Drug-induced cardiomyopathies. Adv. Cardiol. 1974a; 13: 330–348, [PUBMED], [INFOTRIEVE]
- Buja L. M., Ferrans V. J., Rabson A. S. Letter: Unusual nuclear alterations. Lancet March 9, 1974b; 1(854)402–403
- Burhop K. E., Estep T. E. Hemoglobin-induced myocardial lesions. Artif Cells, Blood Subs, and Immob Biotechnology 2001; 29(2)101, Abstract II-4
- Chatterjee R., Welty E. V., Walder R. Y., Pruitt S. L., Rogers P. H., Arnone A., Walder J. A. Isolation and characterization of a new hemoglobin derivative cross-linked between the α chains (Lysine 99α1–Lysine 99α2). J Biol Chem. 1986; 261(21)9929–9937, [PUBMED], [INFOTRIEVE]
- Doherty D. H., Doyle M. P., Curry S. R., Vali R. J., Fattor T. J., Olson J. S., Lemon D. D. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nature Biotechnology 1998; 16: 672–676, [PUBMED], [INFOTRIEVE], [CROSSREF]
- FDA. Summary Basis of Approval. 1973, NDA 17-395, Intropin® (Dopamine Hydrochloride) Injection
- Haft J. I. Cardiovascular injury induced by sympathetic catacholamines. Prog. Cardio. Vas. Dis. 1974; 17: 73–85, [CROSSREF]
- Henry Y., Guissani A. Interactions of nitric oxide with hemoproteins: roles of nitric oxide in mitochondria. Cell. Mol. Life Sci. 1999; 55(8–9)1003–1014, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Linden J. V., Bianco C. Blood Safety and Surveillance. Marcel Dekker Inc., New York 2001; pp. 1–445
- Moreno M., Jr., Nathan L. P., Metze K., Costa S. K., Antunes E., Hyslop S., Zatz R., de Nucci G. Non-specific inhibitors of nitric oxide synthase cause myocardial necrosis in the rat. Clin. Exp. Pharmacol. & Physiol. 1997; 24: 349–352
- Ono Y., Ono H., Matsuoka H., Fugimori T., Frohlich E. D. Apoptosis, coronary arterial remodeling and myocardial infarction after nitric oxide inhibition in SHR. Hypertension 1999; 34: 609–616, [PUBMED], [INFOTRIEVE]
- Patel R. P. Biochemical aspects of the reaction of haemoglobin and NO: Implications for Hb-based blood substitutes. Free Radical Bio. & Med. 2000; 28(10)1518–1525, [CROSSREF]
- Shen W., Xu B. X., Oxhoa M., Zhao G., Wolin M. S., Hintze T. H. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ. Res. 1994; 75: 1086–1095, [PUBMED], [INFOTRIEVE]
- Snyder S. R., Welty E. V., Walder R. Y., Williams L. A., Walder J. A. HbXL99α: a hemoglobin derivative that is cross-linked between the α subunits is useful as a blood substitute. Proc. Natl. Acad. Sci. USA 1987; 84: 7280–7284, [PUBMED], [INFOTRIEVE]
- Walder J. A., Zaugg R. H., Walder R. Y., Steele J. M., Klotz I. M. Diaspirins that cross-link β chains of hemoglobin: bis(3,5-dibromosalicyl) succinate and bis(3,5-dibromosalicyl) fumarate. Biochem. 1979; 18(20)4265–4270
- Zaugg R. H., Walder J. A., Walder R. Y., Steele J. M., Klotz I. M. Modification of hemoglobin with analogs of aspirin. J. Biol. Chem. 1980; 255(7)2816–2821, [PUBMED], [INFOTRIEVE]
- Zhao G., Bernstein R. D., Hintze T. H. Nitric oxide and oxygen utilization: exercise, heart failure and diabetes. Coronary Artery Dis. 1999; 10(5)315–320