Summary
During partial liquid ventilation (PLV) the knowledge of the quantity of exhaled perfluorocarbon (PFC) allows a continuous substitution of the PFC loss to achieve a constant PFC level in the lungs. The aim of our in vitro study was to determine the PFC loss in the mixed expired gas by an absorber and to investigate the effect of the evaporated PFC on ventilatory measurements. Method. To simulate the PFC loss during PLV, a heated flask was rinsed with a constant airflow of 4 L min−1 and PFC was infused by different speeds (5, 10, 20 mL h−1). An absorber filled with PFC selective zeolites was connected with the flask to measure the PFC in the gas. The evaporated PFC volume and the PFC concentration were determined from the weight gain of the absorber measured by an electronic scale. The PFC-dependent volume error of the CO2SMO plus neonatal pneumotachograph was measured by manual movements of a syringe with volumes of 10 and 28 mL with a rate of 30 min−1. Results. Under steady state conditions there was a strong correlation (r2 = 0.999) between the infusion speed of PFC and the calculated PFC flow rate. The PFC flow rate was slightly underestimated by 4.3% (p < 0.01). However, this bias was independent from PFC infusion rate. The evaporated PFC volume was precisely measured with errors <1%. The volume error of the CO2SMO-Plus pneumotachograph increased with increasing PFC content for both tidal volumes (p < 0.01). However for PFC flow rates up to 20 mL/h the error of the measured tidal volumes was <5%. Conclusions. PFC selective zeolites can be used to quantify accurately the evaporated PFC volume during PLV. With increasing PFC concentrations in the exhaled air the measurement errors of ventilatory parameters have to be taken into account.
Introduction
Perfluorocarbons (PFC) were used in respiratory research (Shaffer and Moskowitz, Citation[[1974]]) for more than 30 years. Total liquid ventilation requires high technical expense with specialized developed liquid ventilators (Hirschl et al., Citation[[1995]]) and is currently used in some laboratories for research purposes. Perfluorocarbon associated gas exchange, later called partial liquid ventilation (PLV) was introduced by Fuhrman et al. in the early 90s (Fuhrman et al., Citation[[1991]]). PLV is easy to apply because a conventional ventilator can be used to ventilate the lungs with gas after instillation of PFC into the lung. Therefore PLV found a wider clinical application (Hirschl et al., Citation[[2002]]; Leach et al., Citation[[1996]]). Meanwhile PFC were administered as aerosols (Kandler et al., Citation[[2001]]) and vapor (Ragaller et al., Citation[[2000]]) due to their anti-inflammatory effects as previously described (Koch et al., Citation[[2001]]).
During PLV the instilled PFC is removed from the lung by exhalation (Shaffer et al., Citation[[1997]]). The knowledge of the quantity of evaporated PFC during exhalation (Shaffer et al., Citation[[1997]]) under PLV allows the continuous substitution of the PFC in order to maintain a constant PFC filling volume in the lung. Nevertheless, measurements of the evaporated PFC are rarely performed (Mazzoni et al., Citation[[1999]]; Shaffer et al., Citation[[1997]]) due to technical problems and commercial devices are currently not available. Measuring PFC requires both a specific PFC analyzer and a pneumotachograph to measure the air flow. Depending on the flow sensor used the flow measurement itself depends on the PFC concentration in the breathing gas (Davies and Dunster, Citation[[2000]]; Davies and Dunster, Citation[[2002]]) which is commonly unknown. More easy is the measurements of the total PFC loss by an absorber. We have recently shown that zeolites are highly specific to PFC and allow accurate measurement of PFC volume (Proquitte et al., Citation[[2003]]). The aim of our in vitro study therefore was (1) to validate a simple technique for measuring the evaporated PFC volume during PLV and (2) to investigate the relationship between the PFC content in the breathing gas and the volume error of a commonly available ventilation monitor (CO2SMO+ neonatal monitor).
Methods
Perfluorocarbon
In the current study the perflourocarbon PF5080 (3M Neuss, Germany), a Perfluorooctane (C8F18) was used. PF 5080 has been successfully used for in vitro (Davies and Dunster, Citation[[2002]]) and in vivo experiments (Burkhardt et al., Citation[[2002]]; Foitzik et al., Citation[[2001]]; Gregor et al., Citation[[2003]]) in our laboratory. Its physicochemical properties are different from widely used perfluorooctylbromide (Gabriel et al., Citation[[1996]]), known as “Liquivent” (Alliance, San Diego, USA). PF5080 has a molecular mass of 438 g mol−1 and a boiling point of 101°C. At 25°C PF5080 has a density of 1.77 g (cm3)−1, a dynamic viscosity of 1.4 mPa s, a surface tension of 15 mN m−1 and a vapor pressure of 5.9 kPa.
Description of the Absorber
The zeolite absorber used in this study was described previously (Proquitte et al., Citation[[2003]]). Briefly, it consists of a glass tube with the length of 33 cm and an inner diameter of 6.5 cm filled with 265 g of the zeolite DAY-F63 (Degussa AG, Frankfurt/Main, Germany). It has an air flow resistance of 1.2 cm H2O L s−1 determined by the backpressure at 5 L min−1. DAY-F63 is highly specific to PF 5080 (Proquitte et al., Citation[[2003]]), however, prior to each measurement PFC was again removed from the zeolites by heating (300°C) to utilize the entire capacity of the absorber (48 mL PFC). Provided that the temperature of the absorber was higher than the temperature of the breathing gas there is no adsorption of water vapor.
PFC Measurements
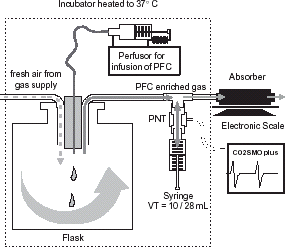
The set-up of PFC measurements by the absorber is shown in . An air flow from a central gas supply was delivered into a sealed flask (50 cm inner diameter, height of 82 cm) in which PFC volumes of 1, 5, 10, or 20 mL were infused with a speed of 3, 15, 30, and 60 mL h−1 using a perfusor with a 20 mL syringe (both Braun Melsungen Ag, Melsungen, Germany). Accuracy of the infused volume by the perfusor is better than 0.02% (data of the manufacturer). Air flow was adjusted by a laboratory rotameter (Aalborg Instruments and Controls Inc., Orangeburg, NY, USA) to 4 L min−1. The set-up was placed within an incubator to keep a constant temperature of 37°C to achieve complete evaporation of the PFC infused, independent of infusion speed. The PFC enriched air from flask streams through the absorber and over a total time of 30 min the weight of the absorber was continuously measured. The scale (LC 2200 P; Sartorius, Göttingen, Germany) has a resolution of 0.01 g, and was connected with a PC using the software Winwedge (V1.1b, T.A.L. Enterprises, Philadelphia, USA). The sampling time of the software was set to 5 s.
Figure 1. Set-up of in vitro measurements to determine absorber accuracy and volume error of the CO2SMO pneumotachograph using a syringe. By modifying the PFC infusion speed the PFC content in the breathing gas could be altered and its influence on volume measurement be analyzed.
After achieving a plateau in weight a total weight difference to the baseline (Δw) was calculated and the PFC volume results by dividing Δw by density. Each measurement was repeated five times.
Ventilatory Measurements
The CO2SMO+ neonatal sensor (Series 3 No. 6720, Novametrix Medical Systems Inc., Wallingford, CT, USA) was used for ventilatory measurements. As shown in , the flow sensor was inserted at the T-piece of the ventilatory circuit and connected with 10 and 100 mL (adjusted to 28 mL) calibration syringes (Hans Rudolph Inc., Wyandotte, MI, USA). Manual movements of the syringes simulated tidal breathing using a rate of 30 min−1. Prior to each measurement a zeroing of the flow sensor was performed as recommended by the manufacturer.
A pilot study was performed to determine the necessary equilibration time after any variation of the PFC infusion speed. Equilibration times up to 10 min were observed. Therefore after adjustment of the air flow and the different PFC infusion speeds (5, 10, and 20 mL h−1) an equilibration period of 15 min was allowed before mean tidal volume was measured over 30 ventilatory cycles. The measurement procedure was repeated with both tidal volumes for all PFC infusion rates for five times.
Statistics
The measurings errors of the adsorbed PFC, the PFC concentration and tidal volume are represented by mean with 95%CI. Statistically significant differences were tested by ANOVA. Correlation analysis was used to investigate the relationship between the measured and the calculated PFC flow rates. The software STATGRAPHICS Plus (Version 5, Manugistics Inc., Rockville, Maryland, USA) was used. As level of statistical significance p < 0.05 was accepted.
Results
PFC Volume Measurement
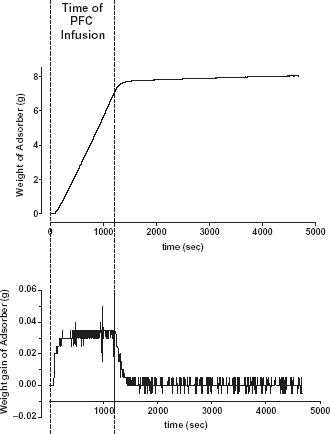
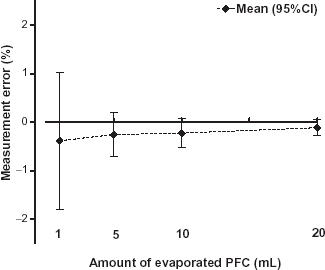
The increase in weight of the absorber after starting the PFC infusion into the flask is shown in (top). In this example a total amount of 5 mL PFC was infused with a speed of 15 mL h−1. After start of the infusion the weight of the absorber rises linearly with a delay time of 3.5 min caused by the transport of the gas through the tubes. With the same delay time, after stop of PFC infusion, a plateau in the weight curve is reached with a minimal steepness. This steepness is likely caused by evaporated PFC stored in the equipment. Therefore, in all measurements the value of the total amount of the adsorbed PFC was taken 10 min after end of infusion of the syringe. The measuring error of the evaporated PFC volume is shown in . The total amount of the PFC volume was slightly underestimated however, the mean deviations were better than −0.5% and the amount of the error decreased with increasing PFC volume.
PFC Flow Measurement
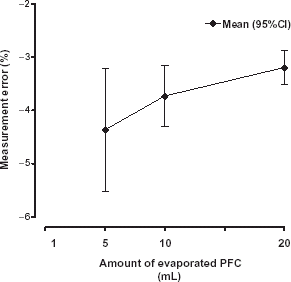
The weight gain (, bottom), calculated by numerical differentiation of the weight curve represents the infusion speed and a plateau is reached within the 10 min time interval. The roughness of the weight gain signal is caused by the digital resolution of the scale. For the lowest infused volume of the perfusor (1 mL with an infusion speed 3 mL h−1) no plateau could be detected due to the noisy signal. Between the infusion speed of the perfusor and the PFC flow rate calculated from the weight gain was a strong correlation (r2 = 0.999). However, the calculated PFC flow is slightly underestimated but did not exceed −5% ().
Volume Error of the PNT as a Function of PFC Content
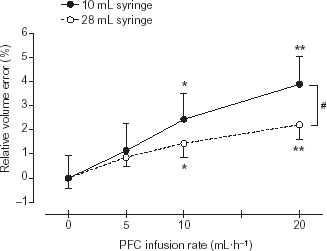
To investigate the influence of the PFC in the breathing gas on the volume measurement of the Pneumotachograph (PNT) (CO2SMO+) the PFC flow rate into the flask was adjusted to 5, 10 and 20 mL h−1 and the background flow was kept constant (4 L min−1). As shown in the relative volume error increased with increasing PFC content for both investigated tidal volumes (10 and 28 mL). This influence of the PFC content was higher for the low tidal volume (10 mL) compared to the large tidal volume (28 mL), with statistically significant difference (p < 0.05) between both volumes for PFC infusion rates ≥20 mL h−1.
Discussion
Our in vitro study has shown that an absorber filled with zeolites can be used as a monitor for continuous substitution of PFC-loss during PLV with volume errors <0.5% provided that the PFC volume during the measuring time is higher than 1 mL. Under steady state conditions the PFC flow rate can be determined by numerical differentiation of the weight gain of the absorber over time. The mean measurement error of the PFC flow-rate was less than 5% for PFC flow-rates >3 mL h−1. Moreover, in this study we have shown that the volume error of the CO2SMO+ neonatal sensor increases statistically significant with increasing PFC flow rates.
PFC Measurements
Measurements of the exhaled PFC during PLV are rarely done probably because it is relatively difficult to measure. Subjective assessment like the “visible meniscus of PFC in the tube” (Hirschl et al., Citation[[2002]]) were used for monitoring the PFC losses. On the other hand, quantitative measurements e.g. by mass spectrometers are to expensive and cumbersome for clinical use. Other gas analytic sensors are currently not commercially available. Shaffer et al. (Citation[[1997]]) developed a mainstream sensor based on thermal conduction and mass transfer of gases. Mazzoni et al. (Citation[[1999]]) used an infrared adsorption analyzer to measure PFC in gases. Both systems allow to measure the PFC concentration. To achieve a PFC mass flow additional measurements of the air flow are necessary. The combination of both a PFC sensor and an air flow sensor in the mainstream can lead to a considerable additive dead space which hampers the ventilation of small lungs. In contrast, our zeolite absorber can be attached at the end of the ventilatory circuit and does not increase the apparative dead space. Therefore this technique seems to be particularly appropriate for measurements in small lungs. However, PFC measurements by an absorber is subjected to methodical limitations. The method is slow and does not allow breath to breath analysis. Furthermore, the duration of measurement is limited by the adsorption capacity of the zeolites and PFC flow measurements are only possible under steady state conditions. Moreover, the drawback that condense water and other volatile substances e.g., anaesthetics (Janchen et al., Citation[[1998]]; Stach et al., Citation[[1995]]) can be adsorbed to zeolites and thus impair PFC measurements must be eliminated. Nevertheless, it is a suitable method to measure evaporated PFC accurately and it can be used to calibrate other PFC sensors due to its precision (see ). Moreover, it is easily available and can be simply adapted to most commonly used ventilators.
Ventilatory Measurement in PFC Enriched Gases
All modern ventilatory strategies require the knowledge of the tidal volume delivered into and from the lungs (Schulze et al., Citation[[2000]], Citation[[2002]]). Davies et al. (Citation[[2002]]) have shown that the presence of PFOB vapor in the breathing gas has a significant impact on ventilation measurements. Thus, calculated parameters of lung mechanics that are commonly used to describe and monitor treatment efficacy are also affected. Our measurements using the CO2SMO+ neonatal sensor have shown that the volume error is PFC dependent and increases nearly linearly with increasing PFC content of the breathing gas. Furthermore, in our measurements the volume error depended on the tidal volume itself. A possible explanation could be the nonlinear characteristic of the CO2SMO+ pneumotach (Roske et al., Citation[[1998]]). Depending on the air flow the changes in the physical properties in the breathing gas may result in different volume errors.
Acknowledgment
The authors would like to thank Betram Foitzik and Ludwik Kurzidim for their assistance and Charles C. Roehr for his linguistic revision. The work was supported by the Grant 01ZZ9511 from the German Ministry of Education and Research.
References
- Burkhardt W., Proquitte H., Krause S., Wauer R. R., Rudiger M. Cerebral oxygenation is affected by filling mode and perfluorochemical volume in partial liquid ventilation of healthy piglets. Biol. Neonate 2002; 82(4)250–256, [PUBMED], [INFOTRIEVE]
- Davies M. W., Dunster K. R. The effect of perfluorocarbon vapour on the measurement of respiratory tidal volume during partial liquid ventilation. Physiol. Meas. 2000; 21(3)N23–N30, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Davies M. W., Dunster K. R. Effect of perfluorocarbon (perfluorooctyl bromide) vapor on tidal volume measurement during partial liquid ventilation. Crit. Care Med. 2002; 30(5)1123–1125, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Foitzik B., Schmidt M., Proquitte H., Schmalisch G. Deadspace free ventilatory measurements in newborns during mechanical ventilation. Crit. Care Med. 2001; 29(2)413–419, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Fuhrman B. P., Paczan P. R., DeFrancisis M. Perfluorocarbon-associated gas exchange. Crit. Care Med. 1991; 19(5)712–722, [PUBMED], [INFOTRIEVE]
- Gabriel J. L., Miller T. F., Jr., Wolfson M. R., Shaffer T. H. Quantitave structure-activity relationship of perfluorinated hetero-hydrocarbons as pot resp. Media: application to oxygen, solubility, log P, viscosity, vapor pressure and density. Asaio-journal 1996; 12: 1–20
- Gregor T., Schmalisch G., Burkhardt W., Proquitte H., Wauer R., Rüdiger M. Aerosolization of perfluorocarbons during mechanical ventilation: an in vitro study. Intensive Care Med. 2003; 29(4)720–726
- Hirschl R. B., Merz S. I., Montoya J. P., Parent A., Wolfson M. R., Shaffer T. H., Bartlett R. H. Development and application of a simplified liquid ventilator. Crit. Care Med. 1995; 23(1)157–163, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hirschl R. B., Croce M., Gore D., Wiedemann H., Davis K., Zwischenberger J., Bartlett R. H. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2002; 165(6)781–787, [PUBMED], [INFOTRIEVE]
- Janchen J., Bruckner J. B., Stach H. Adsorption of desflurane from the scavenging system during high-flow and minimal-flow anaesthesia by zeolites. Eur. J. Anaesthesiol. 1998; 15(3)324–329, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kandler M. A., von der H., Schoof E., Dotsch J., Rascher W. Persistent improvement of gas exchange and lung mechanics by aerosolized perfluorocarbon. Am. J. Respir. Crit. Care Med. 2001; 164(1)31–35, [PUBMED], [INFOTRIEVE]
- Koch T., Ragaller M., Haufe D., Hofer A., Grosser M., Albrecht D. M., Kotzsch M., Luther T. Perfluorohexane attenuates proinflammatory and procoagulatory response of activated monocytes and alveolar macrophages. Anesthesiology 2001; 94(1)101–109, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Leach C. L., Greenspan J. S., Rubenstein S. D., Shaffer T. H., Wolfson M. R., Jackson J. C., deLemos R. A., Fuhrman B. P. Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. The LiquiVent Study Group. N. Engl. J. Med. 1996; 335(11)761–767, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Mazzoni M., Nugent L., Klein D., Hoffman J., Sekins K. M., Flaim S. F. Dose monitoring in partial liquid ventilation by infrared measurement of expired perfluorochemicals. Biomed. Instrum. Technol. 1999; 33(4)356–364, [PUBMED], [INFOTRIEVE]
- Proquitte H., Rudiger M., Wauer R., Schmalisch G. Perfluorocarbon measurements in the breathing gas by an absorber filled with zeolites. Br. J. Anaesth. 2003; 91(5)736–738, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ragaller M., Bleyl J. U., Koch T., Albrecht D. M. From isoflurane to perfluorohexane? Perfluorocarbons–therapeutic strategies in acute lung failure. Anaesthesist 2000; 49(4)291–301, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Roske K., Foitzik B., Wauer R. R., Schmalisch G. Accuracy of volume measurements in mechanically ventilated newborns: a comparative study of commercial devices. J. Clin. Monit. Comput. 1998; 14(6)413–420, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Schulze A. Enhancement of mechanical ventilation of neonates by computer technology. Semin. Perinatol. 2000; 24(6)429–444, [PUBMED], [INFOTRIEVE]
- Schulze A. Respiratory mechanical unloading and proportional assist ventilation in infants. Acta Paediatr. Suppl. 2002; 91(437)19–22, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Shaffer T. H., Moskowitz D. Demand-controlled liquid ventilation of the lungs. J. Appl. Physiol. 1974; 36(2)208–213, [PUBMED], [INFOTRIEVE]
- Shaffer T. H., Foust R., Wolfson M. R., Miller T. F., Jr. Analysis of perfluorochemical elimination from the respiratory system. J. Appl. Physiol. 1997; 83: 1033–1040, [PUBMED], [INFOTRIEVE]
- Stach H., Sigrist K., Radeke K. H., Riedel V. Investigations of the adsorption of halogenated hydrocarbons on microporous adsorbents. Part 3. Gaschromatographic measurement of the initial heat of adsorption Q0 on charcol. Chem. Tech. 1995; 47: 55–61