Abstract
Artificial blood substitutes (ABS) offer an alternative to donated blood. Increasing the oxygen carrying capacity of blood plasma through the addition of a Perfluorocarbon emulsion (PFE) is one approach in creating a blood substitute. The peripheral resistance of the circulatory system may be altered depending on the rheological properties of the ABS. Measurements of the rheological behavior of mixtures of a PFE, OxygentTM, and erythrocyte suspensions were conducted at room temperature at different hematocrits using sheep (nonaggregating) and swine (aggregating) erythrocytes. The pure PFE was found to be shear thinning. Adding 6 and 12 g per 100 mL of sheep blood at the various hematocrits increased the viscosity of the suspension from as low as 4% (6 g PFE/40% Hct) to as high as 26.5% (12 g PFE/plasma). The nonaggregating sheep erythrocyte and PFE mixtures exhibited Newtonian behavior. Shear thinning behavior continued upon addition of 6 and 12 g per 100 mL of swine blood at the various hematocrits, with a slight increase in viscosity in most cases. It was observed that adding 12 g of PFE (≈ 3 × intended clinical dose) to 40% Hct swine blood at room temperature led to a significant decrease in viscosity.
Introduction
The primary physiological function of blood is to carry oxygen (O2) to the cells of the body, and carry away waste products, such as carbon dioxide (CO2). Acute blood loss can lead to severe tissue damage and fatality, but can be compensated through the transfusion of donated blood. Unfortunately, the spread of HIV and the recent increase of the rate of Hepatitis infections have raised concerns about the transmission of disease via the blood supply. The shelf life for donated blood is only six weeks from the time it is collected, which adds significantly to the logistical problems of maintaining a safe and adequate blood supply. Artificial blood substitutes (ABS) may offer an alternative to donated blood and are based on increasing the O2 carrying capacity of plasma. While the phrase “blood substitute” continues to be popular when discussing oxygen-carrying drugs, these products are not intended to replace whole blood, but rather to temporarily perform the gas-transport functions of erythrocytes (Keipert, 1997). They can have a shelf life of about two years and can be produced in sufficient quantity. The effect of intravenous circulation of ABS on blood viscosity, peripheral resistance, and mean arterial pressure needs to be understood before usage.
One approach for achieving the result of increasing the O2 carrying capacity of plasma is the use of a perfluorocarbon emulsion (PFE) administered intravenously (Ivanitskii and Vorob’yev, Citation[[1996]]). Perfluorocarbons are weakly polar compounds in which the solubility of gases increases as their polarity diminishes. These compounds are immiscible in water. Therefore surfactants are required to prepare a stable aqueous-based emulsion which can be injected intravenously. The solubility of O2 in water is only approximately 2% while that in PFE is approximately 50%. The conductivity of O2 rises with an increase in the number of PFE particles, making the erythrocyte a source of high O2 to the tissue (Ivanitskii and Vorob’yev, Citation[[1996]]).
The effect of a PFE on hemorheology and blood trauma has been investigated by Kameneva and coworkers (Kameneva et al., Citation[[1994]], Citation[[1997]]). They showed that the replacement of 20% of the plasma volume (ovine or bovine blood was used for the experiments) with Fluosol, a perfluorochemical that transports O2, reduced mechanical fragility of human and ovine erythrocytes and reduced blood viscosity and erythrocyte sedimentation rate. To our knowledge, sufficient rheological information is unavailable on the effect of PFE concentration on blood of varying hematocrit. The hematocrit may vary in cases of acute blood loss, which maybe either surgically induced or occur in case of an accident. If PFE or mixtures of PFE and whole blood are used under these circumstances, it is important to study the viscosity of the emulsion and/or the mixture. In the closed system of the heart and blood vessels, the working pressure that forces the blood through the variable resistance of the vascular system is the mean arterial blood pressure. This pressure must be closely regulated for two reasons. First, it must be high enough to assure sufficient driving pressure, without which the vital brain and heart do not receive adequate flow, no matter what local adjustments are made in the resistance of the arterioles supplying them. Second, the pressure must not be so high that it creates extra work for the heart and increases the risk of vascular damage and possible rupture of small blood vessels.
The aim of this study was to provide information on viscosity, by studying the rheological behavior of erythrocyte suspensions, the PFE, and mixtures of erythrocyte suspension and PFE at varying hematocrits and varying concentrations of the PFE. The study was conducted on two types of erythrocyte suspensions: one with nonaggregating erythrocytes (Sheep) and the other with aggregating erythrocytes (Swine). The cardiovascular dynamics and architecture of domestic swine shows similarity to humans (Wickham et al., Citation[[1989]]). The RBC count for humans and swine is comparable (4.8 × 106/mm3 for swine and 5.4 × 106/mm3 for humans) (Wickham et al., Citation[[1989]]). Using the above information and the closeness of hematocrit of human and swine blood (47% (Wickham et al., Citation[[1989]]) and 46.3% (Wintrobe et al., Citation[[1981]]), respectively), Wickham et al. (Citation[[1990]]) concluded that the data for swine were comparable to that of humans.
Methods
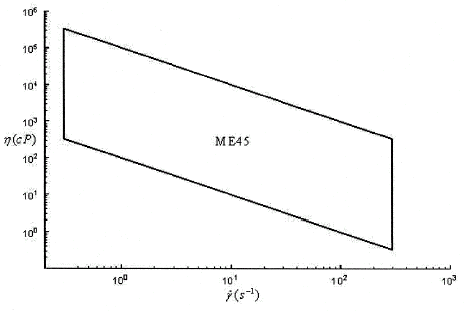
In this study, a concentric cylinder viscometer (Haake CV 20 with ME45 Sensor system) is used to measure the viscosity of mixtures at shear rates from 50 to 300 s−1. The ranges of viscosities that can be measured with this viscometer are shown in . The results presented here are applicable to arteries and veins of the circulation, where blood can be treated as a continuous fluid. Viscosity measurements were performed for a time period of 60 s. A maximum of 1000 data points were collected. The shear rate was increased linearly for 15 s until the desired shear rate was reached and 100 viscosity measurements were recorded. The shear rate was held constant for the following 30 s recording 800 viscosity measurements and the shear rate was ramped down linearly over the remaining 15 s with the remaining 100 measurements being recorded. The viscosity measured for an individual trial is the average of the measurements made over the constant shear rate region. All experiments were conducted at room temperature, which varied between 22 and 24°C.
Figure 1. Ranges of viscosities that can be measured by ME45 Sensor System as a function of shear rate.
The rheometer was calibrated using aqueous solutions of glycerin as the control case. The solutions contained 18 g glycerin and 82 g water (Sample I), 36 g glycerin and 64 g water (Sample II) and 52 g glycerin and 48 g water (Sample III). These mixtures are known to be Newtonian fluids and their viscosity as a function of temperature has been measured (CRC Handbook of Physics & Chemistry, Citation[1998–[1999]]).
Suspensions of sheep and swine erythrocytes mixed with two concentrations of Oxygent, a 60% w/v perfluorocarbon emulsion (PFE), provided by Alliance Pharmaceutical Corp. (San Diego, CA), and were used to prepare samples. Erythrocyte suspensions of hematocrits 0 (plasma) 20, 30, and 40%, were each combined with a dosage of PFE (0, 6, 12 g/dL). Commercially available sheep blood was procured in batches from Quad Five (Ryegate, MT) and swine blood from Cleveland Scientific (Bath, OH), centrifuged (3000 rpm for 15 min) and erythrocytes were re-suspended at the desired hematocrits. In general, 1 dL of each sample was prepared. Further, to make mixtures of erythrocyte suspensions and PFE, 1 dL of erythrocyte suspension was transferred to a container and the required quantity of PFE was added to this container. PFE obtained for the experiments came from two lots of PFE. Experiments on the rheological behavior of pure PFE were conducted using PFE samples from one lot. Experiments on mixtures of PFE and plasma and PFE and erythrocyte suspensions were conducted using PFE only from the second lot. PFE samples from the two lots were not mixed during preparation of mixtures of PFE, plasma, and erythrocytes.
For the experiments, five samples of the desired fluid (aqueous glycerin, PFE, plasma, animal erythrocyte suspensions, and their mixtures) were prepared. Five trials (measurements) were performed at every shear rate on each of the five samples. A reported viscosity, therefore, represents the average of the 25 tests performed for a given fluid. The associated standard deviation of the 25 viscosity measurements for a given mixture is also reported.
Results
The rheological behavior of mixtures of animal erythrocyte suspensions and Oxygent, a known artificial blood substitute were measured to study the effect of artificial blood substitute on aggregation using a concentric cylinder viscometer. Calibration of the viscometer was done by measuring viscosities of aqueous mixtures of glycerin. Suspensions were made with nonaggregating sheep erythrocytes and aggregating swine erythrocytes at hematocrits of 0, 20, 30, and 40%. The current clinically recommended dosage of Oxygent in whole blood is 4 g/dL. The effect of increasing the concentration of PFE to 1.5 and three times the intended clinical dosage was to magnify potential effects and to better resolve trends in the rheological behavior of mixtures. Shear rates of 50, 100, 150, 200, 250, 300 s−1 that were applied to the samples fall within ranges observed in the larger veins and arteries in the circulatory system (Rodkiewicz et al., Citation[[1990]]). The upper and lower limits on shear rate were decided based on capability and sensitivity of the machine. The measured values of average viscosity were fitted, through regression analysis, to the power law model of the form:where η is the measured viscosity (cP),
is the applied shear rate (s−1), a is the consistency index and n is the non-Newtonian index, constants that describe the trend of the viscosity change with shear rate (Walburn, Citation[[1976]]).
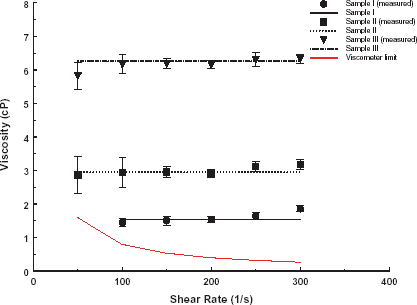
The measured average viscosities of the aqueous glycerin solutions as a function of shear rate are compared with published values (CRC handbook of Physics & Chemistry, Citation[1998–[1999]]) in . The standard deviation decreased with increasing shear rate due to the increase in signal to noise ratio.
Figure 2. Comparison of measured (symbol) and reported (line) viscosities of aqueous solutions of glycerin (Sample I: 18 g glycerin, 82 g water, Sample II: 36 g glycerin, 64 g water, Sample III: 52 g glycerin, 48 g water) over shear rate range 50–300 s−1.
Viscosity measurements made on Oxygent showed that the fluid is non-Newtonian, with a = 60.109 and n = −0.536 (correlation co-efficient = 0.9818). Viscosity measurements made on sheep erythrocyte suspensions without adding Oxygent served as control case. These measurements made at various hematocrit indicated that the suspensions of sheep erythrocytes are Newtonian. A power law model fit for the viscosity measurements yields a non-Newtonian index of less than 0.04 for all samples. Next, rheological behavior of erythrocyte suspensions containing 6 and 12 g of PFE per dL were tested. Average viscosity of sheep erythrocyte suspensions at the different hematocrits and PFE concentrations are summarized in . A power law model fit for the mixtures of 6 and 12 g of PFE with sheep blood at the various hematocrits resulted in non-Newtonian indices of less than 0.02, for all cases, except for the mixture of 12 g of PFE and sheep erythrocyte suspension at 20% Hct, which resulted in a non-Newtonian index of 0.09. Viscosity measurements on mixtures of 6 and 12 g of PFE per dL of sheep erythrocyte suspensions at different hematocrits show that the viscosity of the sample increased with increasing hematocrit and increasing PFE concentration.
Table 1. Average viscosity of sheep blood samples at the different hematocrits and PFE concentrations
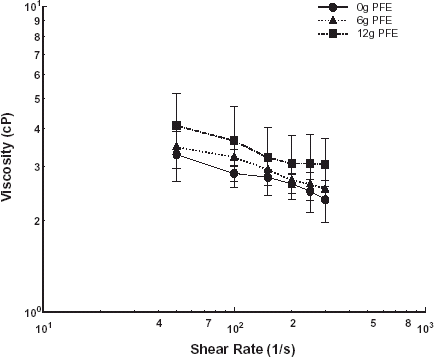
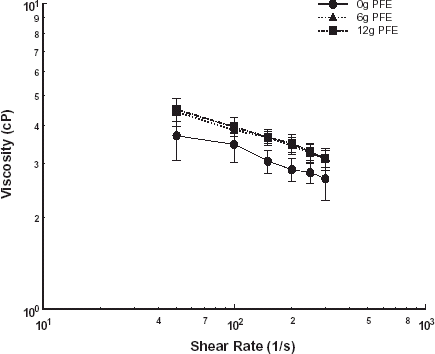
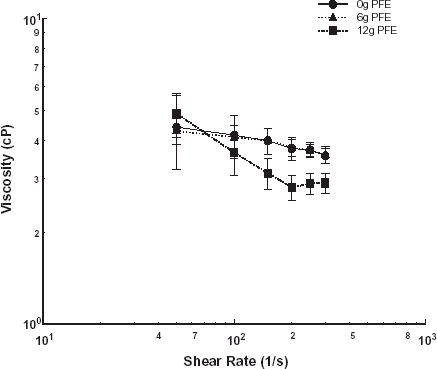
Subsequently, the effect of PFE on the viscosity of aggregating swine erythrocytes was investigated. The power law model was used to represent the shear thinning behavior for the mixtures of PFE and suspensions of swine erythrocytes at varying hematocrit. Viscosity trends of mixtures of 6 and 12 g of PFE per 100 mL of swine blood at 20, 30, and 40% hematocrit are shown in , , , respectively. The consistency indices, a, and the non-Newtonian indices, n, for the power law model fit were obtained by regression analysis and are given in . The non-Newtonian index did not vary significantly with PFE addition at hematocrits of 20 and 30%. At 40% hematocrit, the non-Newtonian index dropped to −0.33 from −0.11 for the control case.
Table 2. The consistency indices and the non-Newtonian indices for the power law model fit for swine blood
Discussion
Viscosity measurements on aqueous glycerin solutions for calibration of viscometer showed maximum errors of 4.7, 0.7, and 1.6% for Sample I, Sample II, and Sample III, respectively. Relatively significant errors (compared to other shear rates) occurred when the viscometer was operated at its maximum capacity of 300 s−1. Errors were higher for lower viscosity samples due to a decrease in the signal to noise ratio. Small differences between the measured values and the reported standard are due to small temperature fluctuations. The results show that the viscometer system is able to measure, with acceptable error, the fluid viscosities in the range expected for the erythrocyte suspension-PFE mixtures.
The observed non-Newtonian shear thinning behavior of pure PFE (a = 60.109 and n = −0.536) is in agreement with that of Ni et al. (Citation[[1992]]) who measured the viscosity of oil–in–water emulsions containing perflubron at up to about 50 volume % and stabilized with egg yolk phospholipid (EYP) at shear rates less than 100 s−1. Viscosities were shear thinning and the magnitude of viscosity was strongly dependent on the volume fraction of perflubron.
Viscosity of sheep erythrocyte suspensions showed Newtonian behavior, as characterized by a non-Newtonian index of less than 0.04 for all samples when fit with a power law model. Laurent et al. (Citation[[1999]]) found viscosity of sheep blood for hematocrits of 24.8 ± 4.5% to be 4.0 cP at 39°C. Viscosity measurement of sheep erythrocyte suspensions at 30% hematocrit at 50 s−1 yielded a value of 3.384 cP at room temperature. A difference of 18.2% in the two values suggests that the effect of temperature may not be very significant on the viscosity of the erythrocyte suspensions. Winderberger et al. (1993–1994) observed nonaggregation of sheep erythrocytes in their experiments and attribute low whole blood viscosity in sheep to this behavior.
A power law model fit for the mixtures of 6 and 12 g of PFE per dL of sheep erythrocyte suspensions at the various hematocrits resulted in non-Newtonian indices of less than 0.02 for all cases, except for the mixture of 12 g of PFE per dL of sheep erythrocyte suspension at 20% Hct, where the non-Newtonian index was 0.09. Such small indices suggest Newtonian behavior. As expected, the increase in viscosity of the mixture when compared to the control case was greater for the 12 g per dL than the 6 g per dL mixture. Mixing relatively small amounts (6 and 12 g/dL) of non-Newtonian PFE with a Newtonian suspension of sheep erythrocytes, resulted in a fluid viscosity of Newtonian behavior.
Viscosity measurements of swine erythrocyte suspensions showed that it was shear-thinning and was in agreement with published data (Amin and Sirs, Citation[[1985]]; Laurent et al., Citation[[1999]]; Windberger et al., Citation[1993–[1994]]). The calculated non-Newtonian indices did not vary significantly over the range of hematocrits of 20 and 30%. At 40% hematocrit, the index increased to −0.11 from −0.19 for the 30% hematocrit sample. This increase is in agreement with Walburn and Schneck (Citation[[1976]]) who state that increase in the non-Newtonian index is due to erythrocyte aggregation at low shear rates. As the hematocrit increases, the number of aggregates increases linking into chains. A change in non-Newtonian index is related to the number of aggregates per unit volume. A difference of 9.5% was found between values measured in our experiments at room temperature (22–24°C) at a shear rate of 50 s−1 and that measured by Laurent et al. at 38.5°C at a shear rate of 50–70 s−1. Suggesting that for this shear rate, the effect of temperature may not be very significant and showing that our measurements for swine erythrocyte suspension without PFE agree reasonably well with published data.
When viscosities of 6 g of PFE per dL of swine erythrocyte suspensions were compared with the control case for swine erythrocytes, a maximum increase of 13% was observed at 200 s−1 and 20% hematocrit. The non-Newtonian index calculated were −0.174 and −0.18 for the mixture and control case, respectively. This suggests that the addition of 6 g/dL of PFE may not have significantly altered the aggregation of swine erythrocytes at this hematocrit. Similar results are also obtained upon comparing the 12 g/dL of PFE per dL of erythrocyte suspensions with the control case, where the maximum increase in viscosity of 24% was observed at 20% hematocrit and 50 s−1 and the non-Newtonian indices were −0.181 and −0.18, respectively. However, addition of 12 g of PFE per dL of swine erythrocytes at 40% hematocrit results in a decrease in non-Newtonian index from −0.117 for the control case to −0.332 for the mixture. This suggests that aggregation of swine erythrocyte is affected at a PFE concentration of 12 g per dL and 40% hematocrit. As the number of perflubron particles and the number of erythrocytes in a given volume of mixture increases, the average distance between a perflubron drop and an erythrocyte decreases. The possibility of increased hydrodynamic interaction between the erythrocytes and perflubron drops may affect the aggregation of erythrocytes that is responsible for the shear thinning behavior of swine blood.
Conclusions
Experiments have been conducted to study the effect of adding Perfluorocarbon Emulsions (PFE)—a known, artificial blood substitute, on the viscosity of nonaggregating sheep erythrocytes and aggregating swine erythrocytes. The measurements show that the mixtures of different dosages of PFE and different hematocrits of blood sample exhibit a complex, rheological behavior.
At a dosage of 6 g of PFE per 100 mL of blood sample, the viscosity of the nonaggregating sheep erythrocyte suspension increases. Sheep blood has a normal hematocrit of approximately 31 ± 7%. The increase in viscosity of the mixture of 6 g of PFE with sheep blood at the lower hematocrit of 20%, results in a viscosity, which is less than sheep blood viscosity at the lower end of normal hematocrit. Similar results are also observed upon addition of 6 g of PFE per 100 mL of sheep blood at 30% Hct. The viscosity of the mixture was less than the viscosity at the higher end of normal hematocrit. This indicates that the effect of adding ≈1.5× the clinically recommended dosage was not significant on the viscosity of the resulting mixture. The peripheral resistance offered by blood vessels in the circulation is directly proportional to the viscosity of the fluid flowing through them. The viscosity of the mixture being less than the control case at higher hematocrit indicates that the peripheral resistance of the blood vessels will not be significantly affected, but, in fact, may be reduced. No increase in the peripheral resistance indicates that the cardiac output does not change to maintain Mean Arterial Pressure. This leads to the conclusion that addition of 6 g of PFE per 100 mL of nonaggregating erythrocyte suspensions would therefore not be expected to affect the cardiac output.
An increase in viscosity is observed upon addition of 6 g of PFE per 100 mL of nonaggregating swine blood. The hematocrit of normal swine blood is 35.6 ± 5.4%. The lower end of the reported normal viscosity of swine blood is 30.2%. The viscosity of mixtures of 6 g of PFE per 100 mL of swine blood at 20% Hct is lesser than the viscosity of the control case at 30% Hct. Therefore, at this lower hematocrit of 20%, addition of 6 g of PFE per 100 mL of swine blood does not increase the viscosity of the mixture to the viscosity of normal swine blood. However, when 6 g of PFE per 100 mL was added to swine blood at 30% Hct, the viscosity compares well with the viscosity of the control case at 40% Hct, but is not higher. According to the reported hematocrit of swine blood, the upper end of hematocrit is 41%. This indicates that the mixture of 6 g of PFE per 100 mL of swine blood at 30% Hct has a viscosity which is comparable to the viscosity of normal swine blood at 40% Hct. Cardiac output to maintain Mean Arterial pressure, therefore, remains the same. This leads to the prediction that addition of 6 g of PFE per 100 mL of aggregating erythrocyte suspensions will not significantly affect the cardiac output.
References
- Amin T. M., Sirs J. A. The blood rheology of man and various animal species. Quarterly Journal of Experimental Physiology 1985; 70: 37–49, [PUBMED], [INFOTRIEVE]
- CRC Handbook of Physics & Chemistry. 79th ed. 1998–1999
- Ivanitskii G. R., Vorob’yev S. I. Organization of motile structures in blood flow—the basis of the function of perfluorocarbon “Artificial Blood.”. Biophysics 1996; 41: 189–201
- Kameneva M. V., Antaki J. F., Konishi H., Whalen J. J., Kerrigan J. P., Watach M. J., Kormos R. L., Griffith B. P., Borovetz H. S. Effect of perfluorochemical emulsion on blood trauma and hemorheology. ASAIO J. 1994; 40: M576–579, [PUBMED], [INFOTRIEVE]
- Kameneva M. V., Borovetz H. S., Antaki J. F., Litwak P., Federspiel W. J., Kormos R. L., Griffith B. P. Effect of perfluorochemical emulsion on hemorheology and shear induced blood trauma. Adv. Exp. Med. Biol. 1997; 411: 383–390, [PUBMED], [INFOTRIEVE]
- Laurent A., Durussel J. J., Dufaux J., Penhouet L., Bailly A. L., Bonneau M., Merland J. J. Effects of contrast media on blood rheology: comparison in humans, pigs, and sheep. Cardiovascular and Interventional Radiology 1999; 22: 62–66, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ni Y., Klein D. H., Pelura T. J. Rheology of concentrated perfluorocarbon emulsions. Biomat., Art Cells & Immob. Biotech. 1992; 20: 869–871
- Peter E., Keipert. Use of Oxygent, a Perfluorochemical-based oxygen carrier as an alternative to intraoperative blood transfusion. 1997, retrieved from www.allp.com
- Rodkiewicz C. M., Sinha P., Kennedy J. S. On the application of a constitutive equation for whole human blood, transactions of the ASME. J. Biomech. Eng. 1990; 112: 198–206, [PUBMED], [INFOTRIEVE]
- Walburn F. J., Schneck D. J. A constitutive equation for whole human blood. Biorheology 1976; 13: 201–210, [PUBMED], [INFOTRIEVE]
- Wickham L. L., Elsner R., White F. C., Cornell L. H. Blood viscosity in phocid seals: possible adaptations to diving. J. Comp. Physiol-B 1989; 159: 153–158, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wickham L. L., Bauersachs R. M., Wenby R. B., Sowemimo-Coker S., Meiselman H. J., Elsner R. Red cell aggregation and viscoelasticity of blood from seals, swine and man. Biorheology 1990; 27: 191–204, [PUBMED], [INFOTRIEVE]
- Windberger U., Ribitsch V., Resch K. L., Losert U. The viscoelasticity of blood and plasma in pig, horse, dog, ox, and sheep. Journal of Experimental Animal Science 1993–1994; 36: 89–95
- Wintrobe M. M., Lee G. R., Boggs D. R., Bithell T. C., Foerster J., Athens J. W., Lukens J. N. Clinical Hematology, 8th ed. 1981; pp. 1891–1899