Abstract
Hemoglobin (Hb)-based oxygen carriers are promising resuscitation fluids for hemorrhagic shock. However, infusion of large amounts of Hb-based material could interfere with reticuloendothelial function potentiating postresuscitation sepsis mortality. We investigated the temporal relationship between hemorrhage-resuscitation and sepsis survival. Male SD rats were subjected to hemorrhage and resuscitated with shed blood volumes of purified human hemoglobin solution (HS). Sepsis was induced by cecal ligation and puncture (CLP) 24 h before, 0, 24, or 72 h after hemorrhage/resuscitation (H/R) and survival was monitored. In additional animals with or without Hb resuscitation, hepatic heme oxygenase-1 (HO-1) gene expression and HO activity were assessed. Seven-day survival for animals resuscitated with HS prior to sepsis induction was significantly higher than other groups. Animals resuscitated with HS showed hepatic HO-1 gene expression while non-HS resuscitated animals did not. In addition, hepatic HO activity levels were significantly higher in HS resuscitated animals than non-HS resuscitated animals. In conclusion, HS resuscitation does not appear to enhance postresuscitation sepsis mortality. Rather, when conducted concomitantly or prior to sepsis, HS resuscitation appears to improve survival from a subsequent sepsis challenge.
Introduction
Hemoglobin (Hb)-based oxygen carriers (HBOC) are promising resuscitation fluids for hemorrhagic shock and are being evaluated in clinical trials (Gould et al., Citation[[1998]]; Kim and Greenburg, Citation[[1998]]). There is, however, a long-standing question as to cell-free Hb impairing host defense mechanisms (Griffiths et al., Citation[[1995]]; Langermans and Bleeker, Citation[[1995]]; Preuett et al., 1985; White et al., Citation[[1986]]). The reticuloendothelial system (RES) is a key and primary defense mechanism against invading pathogenic organisms. It is also a principal site for Hb catabolism. Therefore, infusion of large amounts of HBOC could interfere with the normal host defense functions of the RES. Because many potential recipients of HBOCs may have multiple traumatic injuries, even a moderate compromise in RES function could increase their susceptibility to serious infection. Could HBOC resuscitation enhance the risk of subsequent sepsis and mortality? If it does, little will be gained even if an initial resuscitation with HBOC were a success. Effects of cell-free hemoglobin in blood on host defense mechanisms, however, has not been well studied. In this study, using a rat model of polymicrobial sepsis, we investigated (1) whether resuscitation with cell-free Hb solution would potentiate subsequent sepsis mediated mortality, (2) whether Hb resuscitation has a temporal relationship with sepsis mortality, and (3) possible mechanism involved.
Materials and Methods
Animal Model (Hemorrhage/Resuscitation-CLP)
Male SD rats 250–450 g body weights were anesthetized with sodium pentobarbital (60–65 mg/kg, IP) and the right external jugular vein cannulated for fluid infusion and blood sampling (0.1 mL). After baseline blood sampling, one-third of the estimated blood volume (2.5% of body weight) was withdrawn from the jugular catheter over a five minute period. After a 30 min period of hypovolemic period, the animals were resuscitated with shed blood volumes of purified human hemoglobin solution (HS, 7 g/dL). Sham hemorrhage animals were also cannulated similarly but blood was not withdrawn. Sepsis was induced by cecal lgation and puncture (CLP) 24 h before or 0, 24, and 72 h postresuscitation (N = 5–6 rats for each time point). For chronic experiments, the venous cannula was removed, wound closed with 3-0 silk suture, and the animal returned to cage for monitoring and further experimental procedure at later time. Animals were monitored for survival for 7 days following the sepsis induction. Two additional groups of animals (N = 3 each), one subjected to HS resuscitation as above and the other (control) similarly treated but did not undergo H/R, were killed 24 h later. The liver was harvested and heme oxygenase-1 (HO-1) gene expression and HO enzyme activity were assayed as described below. Animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and the experimental protocol was approved by the Miriam Hospital Institutional Animal Care and Use Committee.
HO-1 Gene Expression
Hepatic HO-1 gene expression was assessed by RT-PCR. Briefly, total RNA was prepared from the liver samples by homogenization-extraction, phase separation, and precipitation using TRI® reagent (MRC, Cincinnati, OH), chloroform, and isopropyl alcohol, respectively. Purified total RNA was reverse transcribed into first-strand cDNAs using MuLV reverse transcriptase (Perkin Elmer, Foster City, CA) according to the manufacturer's protocol. Approximately 10 µL of cDNA product was used for PCR in a reaction mixture containing 0.05 U/L AmpliTaq® DNA polymerase (Perkin Elmer), PCR buffer, 1.5 mM MgCl2, 125 µM NTPs, and 0.05µM each of forward and reverse primers. The primers used were 5′-CACCAGCCACACAGCACTAC-3′ (forward) and 5′-CACCCACC-CCTCAAAAGACA-3′ (reverse) and were expected to produce a 1043 base pair PCR product (Suzuki, Citation[[1999]]). The PCR was performed on a thermal cycler (MJ Research, Watertown, MA) using following thermal cycling protocol; an initial melting for 5 min at 95°C, 35 cycles of 1 min at 95°C, 1 min at 60°C, and 1 min at 72°C followed by a 7 min of final extension. The final PCR products were analyzed on a 1.6% agarose gel premixed with ethidium bromide.
HO Activity Assay
Hepatic HO activity was assessed by a spectrophotometric method based on HO mediated quantitative conversion of heme to biliverdin or bilirubin (8). Briefly, the liver was harvested following in situ perfusion with 0.9% saline. A portion of tissue (200–300 mg) was homogenized in three volumes of 100 mM potassium phosphate buffer (KPBS, pH = 7.4). The crude homogenate was filtered and centrifuged at 600 g for 10 min. The supernate was spun at 10,000 g for 20 min and pellet discarded. The supernate was again centrifuged at 100,000 g for 1 h. The resulting pellet (microsomal fraction) was resuspended in KPBS. The microsomal preparation (400 µL) was incubated with 50 µM hemin and 400 µL KPBS at 37°C for 2 min. 300 µL NADPH (2.5 mM) was, then, added and incubated for another 5 min. HO activity was estimated by measuring absorbance at 640 nm using a HP 4852 spectrophotometer (Hewlett Packard Instruments Co., Palo Alto, CA).
Hemoglobin Solution and Chemicals
The Hb solution used in this study was a purified human Hb (HS; 7 ± 0.5 g Hb/dL) obtained from Hemosol Inc. (Toronto, Canada). Unless otherwise noted, chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Data Analysis
Results are expressed in means ± 1 standard deviation. Statistical significance were assessed using paired t-tests for intragroup comparisons of before and after treatments and unpaired t-tests for intergroup comparisons P < 0.05 are considered statistically significant. For multiple comparisons, analysis of variance (ANOVA) with Neuman–Keuls tests were used. Survival was assessed by plotting Kaplan–Myer survival curves; statistical significance among group survival curves was assessed using a log-rank test at P = 0.05.
Results
HS Resuscitation and Sepsis Survival
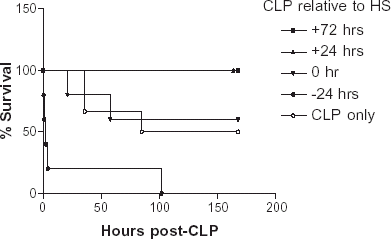
Seven-day survival rate for animals subjected to CLP concomitant with H/R was 67% (N = 6). When H/R was performed 24 h (N = 5) or 72 h (N = 5) before CLP, survival was 100%. In contrast, 0% survival was seen when H/R was performed 24 h after CLP (N = 5). Control sepsis animals (CLP without hemorrhage; N = 6) had a 50% survival. Survival of animals resuscitated with HS prior to sepsis induction was significantly higher than other groups (P < 0.002, Logrank test) (Kaplan–Myer survival curve; ).
Figure 1. Seven day survival curves of animals resuscitated with HS and subjected to a CLP at various time points (24 h before, 0, 24, and 72 h after HS resuscitation). Animals resuscitated with HS prior to sepsis induction had a significantly higher survival rate than other groups (P < 0.002, Log rank test, N = 5–6 each).
HO-1 Gene Expression
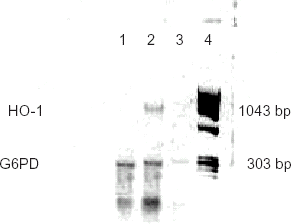
RT-PCR products prepared from hepatic tissue samples from the animals resuscitated with Hb exhibited a band at 1043 base-pair representing a HO-1 gene expression (). HO-1 band was not observed in a negative RT-PCR control (reaction product without MuLV reverse transcriptase) or in tissues from non-HS resuscitated animals.
Figure 2. Hepatic HO-1 gene expressions (RT-PCR) of animals 24 h after HS resuscitation prior to sepsis induction. Lane 1: A RT-PCR product from a non-HS resuscitated animal. A 303 base-pair band represents a control gene (glyceraldehyde-6-phosphate dehydrogenase; G6PDH) expression. Lane 2: A RT-PCR product prepared from a HS resuscitated animal. A 1043 base-pair band indicates HO-1 gene expression. Lane 3: A negative control (RT-PCR done without the MuLV reverse transcriptase). Absence of bands indicates no genomic DNA contamination. Lane 4: DNA weight marker (ΦX174RF/HaeIII fragments).
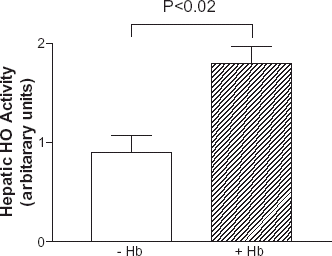
HO Activity
The mean hepatic HO activity levels were significantly higher in HS resuscitated animals than non-HS resuscitated animals (1.8 ± 0.3 U vs. 0.9 ± 0.3 U, respectively, N = 3 each) (P < 0.02, Student's t-test) ().
Discussion
The goals of this study were to investigate whether HBOC resuscitation would enhance sepsis mortality and whether there is a temporal relationship between HBOC resuscitation and sepsis mortality.
The results of this study indicate that mortality of animals resuscitated with Hb depends on the time point when sepsis occurs with respect to hemorrhage-resuscitation. That is, there appears to be a temporal relationship between Hb resuscitation and sepsis survival. If sepsis was induced 24 or 72 h after hemorrhage-Hb resuscitation, 7-day survival was better than control animals. If, however, sepsis was induced 24 h before hemorrhage-Hbresuscitation, there appears to be increased mortality. If sepsis was induced during the hemorrhage-Hb resuscitation procedure, 7-day survival was not significantly different from control animals.
The reason why Hb resuscitation enhanced mortality in postseptic animals is not clear. Even before hemorrhage/Hb resuscitation, these animals could be considered in hypodynamic sepsis phase with depressed cardiac function and systemic hypotension (Wang et al., Citation[[1992]]). In these animals, hemorrhage might have lead to further hemodynamic derangement resulting in decreased tissue perfusion and further deteriorated organ function. It is also plausible that various circulating host defense mechanisms in blood, activated in response to sepsis, might have been lost during the hemorrhage resulting in depressed overall immune function. Of note, hemorrhage alone, in the absence of significant tissue trauma, appears to cause a marked depression of cell-mediated immune function (Ayala et al., Citation[[1994]]). Under this condition, Hb resuscitation might have further diluted the immune factors in blood (leukocytes, complements, antibodies, various intercellular immune signals, etc.) further decreasing the effectiveness of host defense mechanisms. Although, the RES function was not directly assessed in this study, it is not likely that the increased mortality was due to Hb mediated impairment of the RES function because animals resuscitated with Hb prior to sepsis induction showed a better survival rate. The results of this study appear to support previous findings that stabilized Hb solution does not impair RES function but, rather, stimulates overall RE function (Marks et al., Citation[[1994]]). However, the results of this study does not exclude the possibility that acellular Hb in blood could be utilized as a nutritional iron source by invading organisms and requires further studies (Otto et al., Citation[[1992]]). Iron is an essential element required for growth and reproduction by many pathogenic organisms. To date, no direct evidence that HBOC infusion promotes bacterial growth has been reported. Of note, if such Hb utilization by invading pathogen does occur, the short circulation half-time (T1/2) of Hb preparation (90–120 min) used in this study might have contributed to the lower mortality of animals hemorrhaged and resuscitated with Hb 24 or 72 h before sepsis induction. By 24 or 72 h following infusion, most of the infused Hb should have been cleared from circulation, thus, preventing it from being utilized by invading pathogens.
It has been suggested that Hb may enhance toxic effects of endotoxin by forming a complex (Kaca et al., Citation[[1994]]; Su et al., Citation[[1999]]). However, the Hb preparation used in the study was a chemically modified Hb with an unusually high oxidation rate and may not be applicable to other Hb preparations used in HBOC formulations.
The most interesting result obtained from this study is that prior Hb resuscitation appears to render a protective effect on subsequent sepsis mortality. What would be a possible mechanism? One possible explanation could be Hb induction of heme oxygenases (HO), a family of enzymes responsible for catabolism of heme to biliverdin, free iron, and carbon monoxide (CO). While HO-2 is constitutively expressed and functionally active in many cells, HO-1 (also known as a heat shock protein 32) is an inducible isoform that is expressed only in response to certain stimuli. HO-1 activation is believed to be a protective mechanism against stress conditions such as heat, oxidative stress, inflammation, and infection (Kutty and Maines, Citation[[1992]]). Oxidative stress or endotoxin induces HO-1 mRNA expression in cultured cells and whole animals (Carraway et al., Citation[[1998]]; Essig et al., Citation[[1997]]; Yet et al., Citation[[1997]]). Moreover, patients with traumatic injuries or sepsis are reported to have an elevated carbonmonoxy Hb level suggesting HO-1 activation (Monocure et al., Citation[[1999]]). In addition to oxidative stress and infection, hemoglobinemia (caused by hemolysis or exogenous Hb treatment) also induces HO-1. Hb treatment induces HO-1 expression and protein synthesis (Motterlini et al., Citation[[1995]]; Otterbein et al., Citation[[1995]]). Interestingly, pretreatment with Hb protected animals from a lethal endotoxemia (Otterbein et al., Citation[[1995]]) and infusion of Hb preparations to endotoxemic animals resulted in improved survival (Moutelatos et al., 1996).
The mechanism of Hb mediated protection against endotoxemia and sepsis has not been definitively identified but may involve HO-1 induction. Induction of HO-1 by Hb may be beneficial by modulating endotoxin or sepsis mediated proinflammatory responses. HO-1 activation may serve as a protective mechanism against iron catalyzed oxygen radical generation and cellular damage. HO-1 may allow safe recycling of potentially damaging heme-iron (Nath et al., Citation[[1992]]). In addition, HO-1 induction has been reported to downregulate iNOS mRNA expression (Wong et al., Citation[[1995]]) and may modulate the NO mediated hypotension in endotoxemia and sepsis (Hauser et al., Citation[[1996]]). Further, HO-1 deficient cells showed reduced tolerance to stress (Poss and Tonegawa, Citation[[1997]]) and blocking normal HO-1 function with Zn-porphyrin increasesd mortality to sepsis (CLP) (Downward et al., 1997). These findings appear to support the notion that HO-1 induction is a protective (beneficial) mechanism in sepsis and other stress conditions. That HBOCs may have the same effect as native Hb is a potential asset as these products allow broader potential applications.
The results of this study indicate that Hb resuscitation does not enhance subsequent sepsis mortality. Rather, when conducted concomitantly or prior to sepsis occurrence, Hb resuscitation may improve sepsis survival. The improved survival may be mediated through Hb induction of HO-1.
References
- Ayala A., Lehman D. L., Herdon C., Chaudry I. H. Mechanism of enhanced susceptibility to sepsis following hemorrhage. Arch. Surg. 1994; 129: 1172–1178, [PUBMED], [INFOTRIEVE]
- Carraway M. S., Ghio A. J., Taylor J. L., Piantadosi C. A. Induction of ferritin and heme oxygenase-1 by endotoxin in the lung. Am. J. Physiol. 1998; 275: L583–L592, [PUBMED], [INFOTRIEVE]
- Downard P. J., Wilson M. A., Spain D. A. Heme oxygenase-dependent carbon monoxide production is a hepatic adaptive response to sepsis. J. Surg. Res. 1997; 71: 7–12, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Essig D. A., Dorger D. R., Jackson D. A. Induction of heme oxygenase-1(HSP32) mRNA in skeletal muscle following contractions. Am. J. Physiol. 1997; 272: C59–C67, [PUBMED], [INFOTRIEVE]
- Gould S. A., Moore E. E., Hoyt D. B., Burch J. M., Haenel J. B., Garcia J., DeWoskin R., Moss G. S. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J. Am. Coll. Surg. 1998; 187: 113–122, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Griffiths E., Cortes A., Gilbert N., Stevenson P., MacDonald S., Pepper D. Haemoglobin-based blood substitutes and sepsis. Lancet 1995; 345: 158–160, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hauser G. J., Dayao E. K., Wasserloos K., Pitt B. R., Wong H. R. HSP induction inhibits iNOS mRNA expression and attenuates hypotension in endotoxin-challenged rats. Am. J. Physiol. 1996; 271: H2529–H2535, [PUBMED], [INFOTRIEVE]
- Kaca W., Roth R. I., Levin J. Hemoglobin, a newly recognized lipopolysaccharide (LPS)-binding protein that enhances LPS biological activity. J. Biol. Chem. 1994; 269: 25078–25084, [PUBMED], [INFOTRIEVE]
- Kim H., Greenburg A. G. Substitutes for red cell transfusion: 2000–2015. Medicine and Health/Rhode Island 1998; 81: 400–405
- Kutty R. K., Maines M. D. Oxidation of heme c derivatives by purified heme oxygenase. J. Biol. Chem. 1992; 257: 9944–9952
- Langermans J. A. M., Bleeker W. K. Hemoglobin based blood substitutes and infection. Lancet 1995; 345: 864–865, [CROSSREF]
- Marks D. H., Patressi J., Chaudry I. H. Effect of pyridoxalated stabilized stroma-free hemoglobin solution on the clearance of intravascular lipid by the reticulaoendothelial system. Circ. shock 1985; 16: 165–172, [PUBMED], [INFOTRIEVE]
- Monocure M., Brathwaite C. E. M., Samaha E., Marburger R., Ross S. Carboxy-hemoglobin elevation in trauma victims. J. Trauma 1999; 46: 424–427
- Motterlini R., Foresti R., Vandegriff K., Intaglietta M., Winslow R. Oxidative-stress response in vascular endothelial cells exposed to acellular hemoglobin solutions. Am. J. Physiol. 1995; 269: H648–H655, [PUBMED], [INFOTRIEVE]
- Mourelatos M. G., Enzer N., Ferguson J. L., Rypins E. B., Burhop K. E., Law W. R. The effects of diaspirin cross-linked hemoglobin in sepsis. Shock 1996; 5: 141–148, [PUBMED], [INFOTRIEVE]
- Nath K. A., Balla G., Vercelloti G. M., Balla J., Jacob H. S., Levitt M. D., Rosenberg M. E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Invest. 1992; 90: 267–270, [PUBMED], [INFOTRIEVE]
- Otterbein L., Sylvester S. L., Choi A. M. Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am. J. Resp. Cell Mol. Biol. 1995; 3: 595–601
- Otto B. R., Verweij-van Vught A. M. J. J., MacLaren D. M. Transferrin and heme-compounds as iron sources for pathogenic bacteria. Crit. Rev. Microbiol. 1992; 18: 217–233, [PUBMED], [INFOTRIEVE]
- Poss K. D., Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997; 94: 10926–10930
- Pruett T. L., Rotstein O. D., Well C. L., Sorenson J. J., Simmons R. L. Mechanism of the adjuvant effect of hemoglobin in experimental peritonitis IX: the infection potentiating effect of hemoglobin in Escherichia coli peritonitis is strain specific. Surgery 1985; 98: 371–377, [PUBMED], [INFOTRIEVE]
- Su D., Roth R. I., Levin J. Hemoglobin infusion augments tumor necrosis factor response to bacterial endotoxin (lipopolysaccharide) in mice. Crit. Care Med. 1999; 27: 771–778, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Suzuki H. Heme oxygenase-1 gene induction as a intrinsic regulation against delayed cerebral vasospasm in rats. J. Clin. Inv. 1999; 104: 59–66
- Wang P., Zhou M., Rana M. W., Ba Z. F., Chaudry I. H. Differential alterations in microvascular perfusion invarious organs during early and late sepsis. Am. J. Physiol. 1992; 263: G38–G43, [PUBMED], [INFOTRIEVE]
- White C. T., Murray A. J., Smith D. J., Greene J. R., Bolin R. B. Synergistic toxicity of endotoxin and hemoglobin. J. Lab. Clin. Med. 1986; 108: 132–137, [PUBMED], [INFOTRIEVE]
- Wong H. R., Finder J. D., Wasserloos K., Pitt B. R. Expression of iNOS in cultured rat pulmonary artery smooth muscle cells is inhibited by the heat shock response. Am. J. Physiol. 1995; 269: L843–L848, [PUBMED], [INFOTRIEVE]
- Yet S., Pellacanti A., Patterson C., Tan L., Folta S. C., Foster L., Lee W. S., Hsieh C. M., Perrella M. A. Induction of heme oxygenase-1 expression in vascular smooth muscle cells. J. Biol. Chem. 1997; 272: 4295–4301, [PUBMED], [INFOTRIEVE], [CROSSREF]