Abstract
This article describes the immobilization of cholesterol oxidase on a cellulose acetate (CA) membrane activated by Sodium periodate, ethylenediamine, and glutaraldehyde etc. The properties of the immobilized enzyme membrane were investigated. The factors affecting the activity of immobilized enzyme such as the concentration of glutaraldehyde, the concentration of enzyme used during immobilization, temperature, pH, and immobilizing time etc. were also studied. The immobilized COD membrane has been used to construct fibre-optic fluorescent biosensor.
Introduction
Public concern about the risks of high cholesterol levels in blood began to rise in the late 1980s in China. Cholesterol has become one of the main parameters to be determined in routine clinical laboratories. As a consequence, a growing demand for cholesterol biosensor has been noticed in the last few years.
The performance of an enzyme biosensor is ultimately dependent on the ability of its enzymatic membrane and protect enzyme (Robert et al., Citation[[1988]]; Wang et al., Citation[[1995]]). Therefore, it is important to improve the catalytic ability and stability of immobilized enzyme membranes. The method for immobilizing enzymes by using cellulose acetate (CA) has many advantages: the cellulose acetate membrane suitable for immobilizing enzymes is easy to be prepared; immobilized enzyme membrane has better stability; immobilized enzyme has high relative activity (Wang et al., Citation[[1995]]). Therefore, since late 1980s, many researchers had been spending much time on the study of immobilization of enzyme using cellulose acetate membrane. Most of them had been succeeded in applying to biosensor. The literatures about “immobilization of glucose by cellulose acetate for biosensor” were reported one after another, for example, Robert et al. (Citation[[1988]]) led active groups into the membrane by oxidation of periodic acid, then covalently glucose oxidase on the CA membrane, the relative activity of immobilized glucose oxidase was very high, but the method was very complicated and difficult to operate. In this article, on the basis of Sternberg, cholesterol oxidase (COD) was immobilized on the CA membrane by further improving procedure. Moreover, properties of immobilized COD and factors influencing its catalytic activity such as glutaraldehyde, the concentration of enzyme used during immobilization, temperature, pH, and immobilizing time were further discussed. The immobilized COD membrane has been used to construct fiber-optic fluorescent biosensor.
Materials and Methods
Materials
Cellulose Acetate (CA) was purchased from Shanghai Chemical Reagents Purchasing Station; Cholesterol oxidase (COD) was purchased from Merk Co., Ltd. (Germany); Horseradish peroxidase was purchased from Spromega (Shanghai, P.R.C.); Phenol (Analytical grade) was purchased from Wuhan Chemical Reagents Factory (Wuhan, P.R.C); 4-Aminoantipyrine (Analytical grade) was purchased from Wuhan Pharmaceutical Factory (Wuhan, P.R.C.); 25% Glutaraldehyde solution was purchased from Merk Co., Ltd. (Germany); Ethylenediamine (Analytical grade) was purchased from Chemical Plant of Hubei University (Wuhan, Hubei); Sodium periodate (Chemical grade) was purchased from Chemical Reagents Factory of Tianjin University (Tianjin, P.R.C.); Ru(Phen)3Br2 (Analytical grade) was obtained from Professor Zhike He of Wuhan University. All other reagents are analytical grade.
Apparatus
751-GW Spectrometer (Shanghai Analytical Instrument Factory, P.R.C.); LRH-150B Incubator (Guang-dong Medical Device Factory, P.R.C.), 89HW-1 Thermostat & Magnetic Stirrer (Lecheng Electrical Equipment Factory, P.R.C.); Fiber-optic fluorescence detector (Made in Wuhan University of Technology).
Immobilization of COD
Cellulose Acetate Membrane Activation
Referring to literature (Robert et al., 1988; Wang et al., Citation[[1995]]), in this article, the procedure of membrane activation was as followed: the cellulose acetate membranes of defined thickness (30 µM) were immersed into 0.2 mol/L sodium periodate solution for half an hour; then the membranes were washed with 0.02 mol/L pH 7.0 phosphate buffer solution (PBS) and immersed in 10 mL of 0.1 mol/L ethylenediamine solution for 2 h at 25°C; after washed in distilled water for 5 min, the membranes were activated by treatment with 1 mL of a 2.5% glutaraldehyde solution for 2 h, which led aldehyde groups in the membranes.
Immobilization of COD on the Activated CA Membranes
After washing of 5 min in distilled water, the activated CA membranes were immersed into seven kinds of COD solutions—1, 2, 4, 6, 8, 10, 12 mg/100 µL respectively, and left to cross-linking for 4 h at 25°C, then the membranes were washed with 0.05 mol/L phosphate buffer solution and stored in the PBS at 4°C when not in use.
Measurement of Enzyme Activity
The catalytic activity of immobilized COD was measured as followed (Wang et al., Citation[[1995]]): First, 1 mL of 2.4% phenol solution, 3.5 mg 4-aminoantipyrine and 3.5 mg horseradish peroxidase were added to 20 mL of 0.02 mol/L pH 7.0 phosphate buffer solution and homogenized, which was called Solution1; Solution2 was 5 mmol/dL cholesterol solution. Second, the COD CA membrane (1 cm × 1 cm) was put into 20 mL substrate solution (mixture of solution1 and solution2) with stirring at constant temperature; at last, 2 mL reacting solution was taken out to measure its absorbance at A500 every 5 min.
Application of Immobilized Membrane: Construction of Biosensor
In this article, the fiber-optic fluorescent biosensor was constructed based on cholesterol oxidase (COD), which could catalyze the following reaction (Allain et al., Citation[[1974]]; Fernandez et al., 1987; Robert et al., Citation[[1988]]):where the amount of O2 consumed during enzymatic reaction is positively relevant to that of cholesterol, Ru(Phen)3Br2 could emit fluorescence at irradiation of 485 nm light, which could quench while Ru(Phen)3Br2 contact with O2, the decrease of fluorescence intensity and life time of the sensor material as a function of O2 (Carraway et al., Citation[[1991]]), when the samples contact with biosensor, as dissolved oxygen is consumed in the course of the enzymatic reaction, an increase of fluorescence from immobilized Ru(Phen)3Br2 is measured at 620 nm (λexc, 485 nm) and can be related to the cholesterol concentration in the sample, therefore, the concentration of free cholesterol in serum or blood could be measured indirectly.
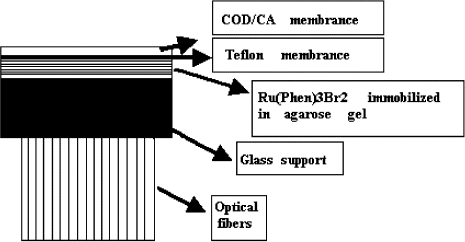
shows a schematic diagram of the fiber-optic fluorescent biosensor membrane, the sensitive membranes comprise three different layers: one containing cholesterol oxidase immobilized on the CA membrane, the other one is Tefron membrane preventing fluorescent leakage, the last layer which acts as the oxygen optosensor that incorporates the Ru(Phen)3Br2 embedded in agarose gel.
Results and Discussion
Effect of the Concentration of Glutaraldehyde on the Activity of Immobilized COD
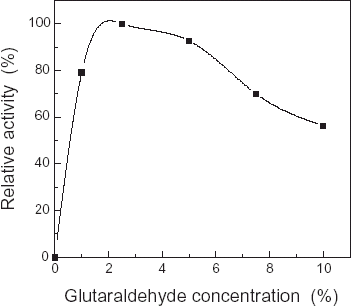
The cellulose acetate membranes prior to be treated by 0.1 mol/L ethylenediamine solution were immersed into 1, 2.5, 5, 7.5, 10% glutaraldehyde solution respectively to react for 1 h, then washed with 0.05 mol/L pH 7.0 phosphate buffer solution, next, these membranes activated were immersed into the COD solution of 8 mg/100 µL to cross-link for 4 h. In the end, immobilized COD membrane was washed with 0.05 mol/L pH 7.0 phosphate buffer solution and their activity was measured and shown in . As can be shown in , while the concentration of glutaraldehyde was lower (<2.5%), the relative catalytic activity of immobilized COD increased significantly with the increment of glutaraldehyde concentration; at 2.5% glutaraldehyde solution, the relative catalytic activity of immobilized COD reached high peak, then, it decreased slowly with the increment of glutaraldehyde concentration.
Effect of the Amount of Enzyme used during Immobilization on the Activity of Immobilized COD
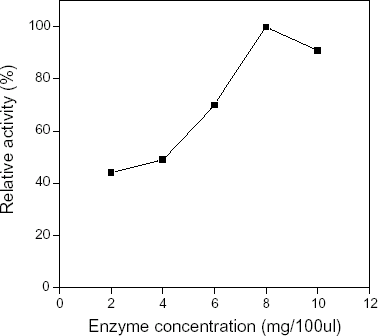
Cellulose acetate membranes activated by 2.5% glutaraldehyde solution were immersed into seven different concentration of COD solutions—1, 2, 4, 6, 8, 10, 12 mg/100 µL respectively to cross link for 4 h, then washed with 0.02 mol/L pH 7.0 phosphate buffer solution, after that, their catalytic activity was determined. The results were shown in , it was observed that the relative activity of immobilized COD depended directly upon the concentration of enzyme used during immobilization in the range 1–8 mg/100 µL, at 8 mg/100 µL, the relative activity was 100%; then, the relative activity of COD depended inversely upon the concentration of COD used during immobilization. Chen et al. (Citation[[1996]]) and Wang et al. (Citation[[1995]]) explained this fact when they studied “immobilized cellulase” and “immobilized glucose oxidase” as followed: the amount of activated aldehydic groups per unit volume of cellulose acetate was constant. While the combining site were not saturating, the relative catalytic activity of immobilized COD increased with the concentration of enzyme used during immobilization increasing; when the combining sites had been saturated, the catalytic activity didn’t increase with the increment of the concentration of enzyme used during immobilization. This explanation seemed to be reasonable, but it could lead people onto a wrong path. The amount of enzyme used during immobilization was equal to that of enzyme immobilized on the CA membrane. In fact, the amount of enzyme immobilized on the CA membrane were several µg per square centimeter, only a little enzyme was coupled on the CA membrane during immobilization. Therefore, the explanation mentioned above were not reasonable, which could not expound the mechanism of enzymes immobilization. In this article, we believe in that the main reasons resulting in the fact were: the COD in solution could be immobilized on the CA membrane only by covalently combining with the active aldehydic groups in CA membrane, if not, COD could not be immobilized on the cellulose acetate membrane. In diluter COD solution, there were fewer COD molecules in per unit volume, which resulted in fewer COD molecules could be immobilized on the CA membrane per unit time. In the concentrated COD solution, there were more COD molecules in per unit volume and more COD molecules could move to the surface of CA membrane per unit time, which led to the result that more COD molecules were immobilized on the CA membrane per unit time. Therefore, the relative activity of immobilized COD was more higher. In other words, the concentration of enzyme used during immobilization determined the amount of COD molecules moving to the surface of CA membrane and dominate the amount of COD molecules immobilized on the CA membrane, which control the relative catalytic activity of immobilized COD membrane ultimately. In order to demonstrate our idea, during immobilizing COD in diluter COD solution, the time of immobilization was extended appropriately compared with the concentrated solution used, as a result, the relative catalytic activity of COD increased significantly. As to the concentration of COD used during immobilization exceeded 8 mg/100 µL, the relative catalytic activity of immobilized COD membrane depended inversely upon the amount of COD enzyme used during immobilization. In this article, we thought it was because excessive COD molecules immobilized on the surface of CA membrane hindered COD moving freely in the domain of space so that decreased the relative activity of COD membrane.
Effect of Temperature on the Activity of Immobilized COD
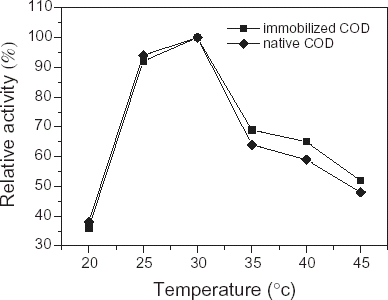
A temperature-related profile was studied in native cholesterol oxidase and its immobilized derivatives. The enzyme-immmobilized membrane was kept at different temperatures, from 20 to 45°C, for 60 min. As can be seen in , the activity of COD was more sensitive to temperature at pH 7.0, 5 mmol/dL of cholesterol solution, optimal temperature of immobilized COD was 30°C. During 20–30°C, the catalytic activity of immobilized COD was dependable directly upon a rise in temperature, this value was slightly lower than the one described in the literature for the optimum performance of cholesterol oxidase solution (Krug et al., Citation[[1992]]) and slightly higher than the one described by Marazuela et al. (Citation[[1995]]). The optimal temperature of enzyme was related to the concentration of substrate, medium pH, strength of ion and time of incubation (Norma and Nakayama, Citation[[1976]]). Therefore, the value may be related to the time of incubation. But compared to literature (Hemant et al., Citation[[1999]]), their optimum temperature was 10°C lower than that of immobilized COD we used in experiments, this may be connected with the bacteria species being used to extract COD.
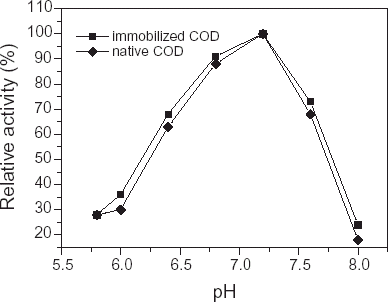
Effect of pH on Activity of Immobilized COD
The activity profile was investigated under membrane storage conditions. The immobilized enzyme was kept in different pH buffer solutions (5.8–8.0) for 60 min. As can be seen in , the peak value was obtained at pH 7.2, the optimal pH value for the enzyme activity in homogeneous solution has been reported to be 7.0 (Wolfgang and Otto, Citation[[1990]]), compared with this value, the immobilized enzyme resulted in a shift to a slightly more alkaline pH. In addition, in the range pH 5.8–6.4 and pH 7.6–8.0, the activity of immobilized COD was lower, probably due to weak acid environment and weak base environment around the COD causing partial enzyme's inactivation. Hemant (Citation[[1999]]) reported the immobilized COD was stable from pH 6 to 7.5, the difference may be related to the bacteria species being used to extract COD and matrice used in the experiment.
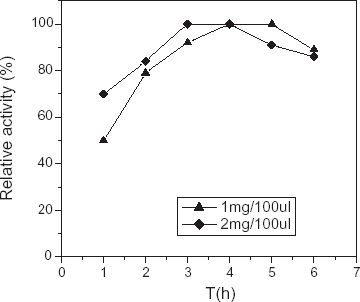
Effect of Immobilization Time on the Activity of Immobilized COD
As to the problem of immobilization time i.e., how long could obtain the optimum activity. Many scholars drew different conclusions: 6 h; 24 h (Krug et al., Citation[[1992]]; Wolfgang Oho, Citation[[1990]]); more than 6 h (Hemant et al., Citation[[1999]]). In this article, we thought that the reason causing different results mentioned above may be related to several factors as followed:
The matrix and method activating matrix. Different matrix and activating methods can form different active groups having different activity which influenced the velocity of active groups combining with COD molecules.
The concentration of COD used during immobilization. While the concentration of COD during immobilization was higher, the shorter time for immobilizing enzyme could make CA membrane obtain higher relative catalytic activity. On the contrary, while the amount of COD during immobilization were lower, it could also improve the catalytic activity of COD by prolonging immobilization time appropriately.
Temperature. Generally, immobilized enzyme membrane needed longer time to obtain higher relative activity at lower temperature; on the contrary, it needed only shorter time. In order to demonstrate our conclusion, we investigate the relationship between the immobilization time and the catalytic activity of immobilized COD using different concentration of COD solution in 1 and 2 mg/100 µL at 25°C. As can be shown in , at limited range, the relative catalytic activity of immobilized COD was positively relevant with immobilization time.
Long-term Stability
When not in use, COD membrane were kept in 0.05 mol/L pH 7.0 PBS and stored in refrigerator at 4°C. The catalytic activity of COD membrane was determined at 1, 2, 3, 4, 5 weeks respectively. At 5th week, COD membrane kept 90% of their original activity.
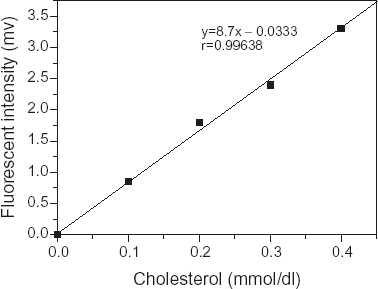
Response of Fiber Fluorescent Cholesterol Sensor
When the fiber fluorescent cholesterol sensor came into contact with cholesterol solution, the sensor response to the different concentrations of cholesterol was almost linear, as shown in .
Conclusions
Factors affecting the catalytic activity of immobilized COD such as the concentration of glutaraldehyde, temperature, and pH etc., were very important for improving the relative activity and stability of CA memrane. Some conclusions were drawn: Concerning to all kinds of factors, in order to obtain the catalytic activity of immobilized COD, optimal conditions were: (i) the concentration of glutaraldehyde was 2.5%; (ii) the concentration of COD used during immobilization was 8 mg/100 µL; (iii) the time of immobilization was 4 h at 25°C; (iv) optimal pH was 7.2.
Acknowledgments
This work is supported by “the Research Fund for the Doctoral Program of Higher Education” of P.R. China.
References
- Allain Ch. C., Poon L. S., Chan C. S. G, Richmond. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974; 20(4)470–474, [PUBMED], [INFOTRIEVE]
- Carraway E. R., Demas J. N., DeGraff B. A., Bacon J. R. Photophysics and photochemistry of oxygen sensors based on luminescent transition-metal complexes. Anal. Chem. 1991; 63: 337–342
- Chen Sheng, Huang Zhiyue, Liu Yanru. Study on cellulase immmobization on chitosan. Prog. Biochem. Biophys. 1996; 23(3)250–253
- Fernandez-Romero J. M., Luque deCastro M. D., Valcarcel M. Determination of total cholesterol in serum by flow injection analysis with immobilized enzymes. Clin. Chim. Acta 1987; 167: 97–104, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Gu Tianjue, Feng Zongchen. Biochemistry. Beijing People Medical Publishing House. 1997; 59 pp.
- Hemant Kumar, Ashok Kumar, Poonam Kumari, Jyotirmai S., Tulsani N. B. Immobilization of cholesterol oxidase on formvar using organic solvents. Biotechinol. Appl. Biochem. 1999; 30: 231–233
- Krug A., Suleiman A. A., Guilbault G. G. Enzyme-based fiber-optic device for the determination of total and free cholesterol. Anal. Chim. Acta 1992; 256: 263–268, [CROSSREF]
- Marazuela M. D., Cuesta B., Moreno-Bondi M. C., Quejido. Free cholesterol fibre-optic biosensor for serum samples with simplex optimization. Biosens. Bioelectron. 1995; 12(3)233–240, [CROSSREF]
- Norma A., Nakayama K. Polarographic method for rapid microdetermination of cholesterol with cholesterol esterase and cholesterol oxidase. Clin. Chem. 1976; 22(3)336–340
- Sternberg, Robert, Dilbir S., Bindra, George S., Wilson, Thevenot, Daniel R. Covalent enzyme coupling on cellulose acetate membranes for glucose sensor development. Anal. Chem. 1988; 60: 2781–2786, [PUBMED], [INFOTRIEVE]
- Wang Shunguang, JiXinsong, Yuan Zhongyi. Study of cellulose acetate membrane-based glucose biosensor. Chin. J. Biotech. 1995; 11(3)260–265
- Wolfgang Trettnak, Otto S. Wolfbeis. A fiberoptic cholesterol biosensor with an oxygen optode as the transducer. Anal. Biochem. 1990; 184: 124–127