Abstract
Catalase enzyme (EC 1.11.1.6) was immobilized by entrapping in cellulose acetate beads. This organic matrix is highly resistant to mechanical stability and can be used under various conditions. Initial studies were conducted to examine the immobilization ability of catalase on the matrix previously activated with a series of reagent normally and the best results were obtained with the beads activated with Ce(SO4)2. In the optimization studies of the immobilized enzyme optimum pH and temperature were found as pH:7.0 (Tris–HCl, 50 mM) and 35°C. In the characterization studies of the immobilized enzyme some parameters such as storage and thermal stability were investigated. Finally, the immobilized enzyme was used for the decomposition of hydrogen peroxide in milk samples and also by using a catalase biosensor prepared the decomposition level of hydrogen peroxide was detected.
Introduction
Using of enzymes in nature form for biochemical, biotechnological, biomedical, and the other areas such as environmental, food industries is not generally suitable for both economical and the product yield. So, immobilization of enzymes by using some different chemical and physical methods and using them in the immobilized form especially in medicine, industry, and biotechnological process is the most widespread way (Çetinus and Öztop, Citation[[2003]]; Mosbach, Citation[[1988]]; Tarhan and Uslan, Citation[[1990]]). Hydrogen peroxide is very important agent and commonly used in many industrial and biotechnological process for waste water treatment and sterilization (Tarhan and Akertek, Citation[[1995]]). In the diary industry it is used for cold pasteurization of the milk as an antibacterial agent to prevent the microbial contamination. So, determination of hydrogen peroxide is a very important and necessary for human health. For this purpose some methods based on HPLC (Nakoshima et al., Citation[[1994]]), enzymatic-spectrophotometric (Demmano et al., Citation[[1996]]), and also biosensors (Akgöl and Dinçkaya, Citation[[1999]]; Wang et al., Citation[[1995]]; Stein and Hain, Citation[[1995]]) were developed. Hydrogen peroxide is substrate of catalase enzyme and it is used in the diary process in order to prevent microbiological contamination. At the end of the process all of the hydrogen peroxide is degraded into O2 and H2O by catalase enzyme. So the immobilization of catalase is very important for both economical and yielded usage of the enzyme.
The aim of the present work was to immobilize the catalase by entrapping in cellulose acetate beads. For the optimization of the immobilization procedure some parameters such as effects of pH and temperature were investigated. The immobilized enzyme was used for the decomposition of hydrogen peroxide in milk samples and also by using a catalase biosensor prepared according to Akgöl and Dinçkaya (Citation[[1999]]) the decomposition level of hydrogen peroxide was detected. In this method dissolved oxygen concentration which was related to hydrogen peroxide decomposition determined by using a YSI 57 Model oxygenmeter.
Materials And Methods
Materials
Catalase [E.C 1.11.1.6] from bovine liver, cellulose acetate, bovine serum albumin (BSA), and glutaraldehyde (25%) were obtained from Sigma (St. Louis, USA). Hydrogen peroxide (30%), Ce(SO4)2, and all other chemicals used in the experiments were purchased from Merck (Darmstadt, Germany).
Apparatus
In the spectrophotometric analyses a Jasco V-500 uv/vis spectrophotometer (Japan) were used. In the biosensor experiments a YSI 57 Model oxygenmeter (YSI, Yellow Springs, Ohio) combined with a YSI 5739 Model dissolved oxygen (DO) probes based on amperometric consist of Au (cathode), Ag-AgCl (anode), half saturated KCl (electrolyte), a teflon membrane which is selective for oxygen, and an ultrathermostat (Colora, Germany) were also used.
Preparation of Cellulose Acetate Beads
For this purpose 900 mg cellulose acetate was dissolved in 5 mL of acetone and then the solution was added drop-wise into hegzane solution by using a suitable syringe at 25°C.
Immobilization of Catalase in Cellulose Acetate Beads
Catalase was immobilized in cellulose acetate beads prepared by using both entrapping and cross-linking processs together. For the immobilization of catalase 700 piece of beads (approximately 1 g) were added to 100 mM of Ce(SO4)2 solution prepared in 0.1 N HNO3 with stirring for 2 h. At the end of the time, the beads were removed from the solution by a decantation process and washed with distilled water. And then the beads were added to 10 mL of BSA solution (10 mg/mL) prepared in borate buffer (pH:9.0, 0.1 M) and stirred again for 30 min. 40 mg of sodium cyanidborhydrid was added to the solution and continued to stirring for 2 h. The beads were treated decantation process again and washed with distilled water. After this step the beads were treated with 2.5 mL of catalase enzyme solution (11200 U/mL) and stirred for 10 min. Glutaraldehyde solution (2.5%) was added to the mixture and continued to stirring for 5 min. Finally, the beads were removed from the mixture by a decantation process and washed.
Activation of the Cellulose Acetate Beads by Ce(SO4)2
In the activation of the cellulose acetate beads prepared, catalase was bound to cellulose acetate beads by covalent bonds. In this case, hydroxyl groups of cellulose acetate were oxidized to aldehyde groups by Ce(SO4)2 (0.1 M prepared in 0.1 M HNO3) and activated. After activation step, spacer arms were formed by occurring Schiff bases between aldehyde groups and –NH2 groups of BSA. Catalase and BSA were immobilized together by using a cross-linking agent, glutaraldehyde.
Measurement of Free and Immobilized Catalase Activity
Activity mesurement of free and immobilized catalase was made for 10.5 mM hydrogenperoxide at 240 nm by using a uv/vis spectrophotometer according to Aebi (Citation[[1974]]). Activity of catalase immobilized was accepted as µmol/dk g (immobilized beads). The molar extinction coefficient is 40 cm2/µmol at this wavelength.
Preparation of Catalase Biosensor
Catalase (9324.2 IU/mL) and 225 bloom gelatin (33.3 mg/mL) were mixed at 38°C in distilled water. Two hundred microliters of the mixed solution was spread over dissolved oxygen (DO) probe membrane and allow to dry at 4°C for 1 h. Finally, it was immersed in 2.5% glutaraldehyde in 50 mM phosphate buffer (pH:7.5) for 3 min. The biosensor prepared contained 1650.3 IU/cm2 and 5.9 mg/cm2 gelatin in the bioactive layer.
Results and Discussion
Optimization Studies
In this section of the study effect of pH; temperature, buffer system, and buffer concentration on the free and immobilized catalyzes were investigated.
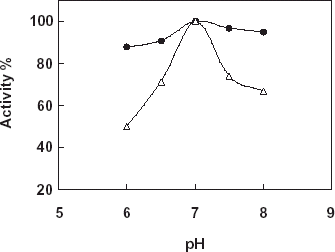
Effect of pH
For the determination of the effect of pH on the free and immobilized catalase phosphate buffers (50 mM) that is in the pH range 6.0–8.0 were used. shows the results obtained. According to the figure the highest activity was obtained in pH:7.0 for free and immobilized catalase. Therefore, the pH profile of the immobilized catalase was much broader with respect to the free enzyme. By using different buffer systems such as Tris–HCl, phosphate, and phosphate/citrate (pH:7.0, 50 mM) the most suitable buffer system for the enzyme activity was determined. Results obtained from the experiments showed that phosphate buffer system gave the most suitable results for both free and the immobilized catalase. After detection of the most suitable buffer system phosphate buffers at varying concentrations (25, 50, 100, and 200 mM) were prepared and tested for the maximum enzyme activity. According to the results, although free enzyme shown the maximum activity at 100 mM immobilized catalase shown the maximum activity when 50 mM concentration of the buffer used.
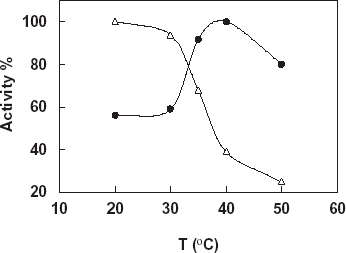
Effect of Temperature
Determination of the effect of temperature on free and the immobilized catalase activity was investigated in phosphate buffer in a temperature range 10–40°C ().
Figure 2. Effect of temperature on free and immobilized catalase, -Δ-: free catalase, -•-: immobilized catalase (pH 7.0: phosphate buffer, 50 mM).
From the figure it can be said that the optimum temperature for both free and immobilized catalase in the working conditions is found as 35°C. This is the optimum temperature of free enzyme given in literatures and enzyme handbooks so immobilization procedure didn’t show an effect on the optimum temperature of the enzyme. There was no important observation in activity change of free enzyme by increasing of the temperature. Therefore, increase in the temperature resulted in an activity increase for the immobilized enzyme.
Characterization Studies
In order to investigate characterization of the free and immobilized catalase enzyme some parameters such as thermal and pH stability experiments were done.
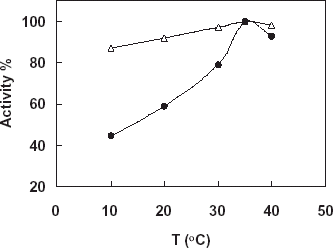
Detection of Thermal Stability of the Free and Immobilized Catalase
In this study the free and immobilized catalase were incubated in phosphate buffer (pH:7.0, 50 mM) at different temperature (20–50°C) for 18 h. At the end of the time under the optimum conditions the activities of the enzymes were assayed. shows the results obtained. According to the figure increase temperature from 20 to 40 resulted in increase in activity of the immobilized enzymes, so it can be said that immobilization was gained a thermal stability to the enzyme related to increase its conformational stability by the immobilization. On the other hand, there was a negative effect in the activity of free enzyme by increase in the temperature.
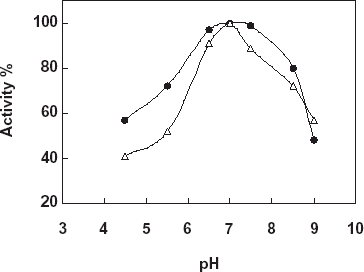
Detection of pH Stability of the Free and Immobilized Catalase
For the determination of pH stability free and immobilized catalase were incubated in buffers that is in the pH range 4.5–9.0 for 18 h at 20°C. At the end of the time the activity measurements of the enzymes were made under the optimum assay conditions (). As it is shown from the figure pH stability of the both enzymes shown a similarity. Both free and the immobilized catalase gave an optimum at pH:7.0, however, the immobilized catalase gave a much broader pH stability between pH:6.5 and pH:7.5 with respect to the free enzyme. The results obtained can be thought as an effect of micro environment of the enzyme and pH on the enzyme stability.
By using Catalase Biosensor Determination of Hydrogen Peroxide Decomposition in Milk Samples
In the experiments a YSI Model 57 oxygenmeter and 5739 Model dissolved oxygen probe covered with a oxygen sensitive teflon membrane were used. Biosensor measurements were done in the steady-state condition at 35°C. Milk samples contained 0.03 and 0.1% hydrogen peroxide were prepared. Before the treatment of the milk samples by the immobilized catalase the hydrogen peroxide concentration of the samples (0.03 and 0.1%) were determined by using the catalase biosensor prepared. The milk samples were treated with the immobilized catalase (450 beads obtained 27.39 U/50 mL) for 70 min by stirring. During this time, in the beginning and per 20 min samples treated with immobilized catalase were taken from the reaction medium and hydrogen peroxide decomposition level of the milk samples were determined by using catalase biosensor. Addition of the milk samples contained hydrogen peroxide in to the reaction cell caused a rapid current increase due to hydrogen peroxide destruction in the enzyme layer on the biosensor (). According to the results given in the figure, it was detected that 78% of hydrogen peroxide of sample 1 (0.03%) and 22% of hydrogen peroxide of sample 2 (0.1%) decomposed in first 10 min. During from 10 to 70 min there was no change in the decomposition level for sample 2. This results from catalase enzyme denaturation occurred in high concentration of hydrogen peroxide. On the other hand, at the end of the 70 min all hydrogen peroxide of sample 2 was decompozed.
Figure 5. Determination of decomposition level of hydrogen peroxide in milk samples by using the catalase biosensor, -•-: Sample 1 contained 0.03% H2O2, -Δ-: Sample 2 contained 0.1% H2O2.
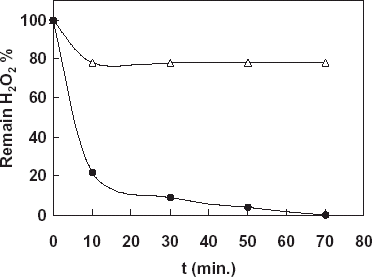
From all the results obtained from the biosensor it is obvious that catalase enzyme immobilized in cellulose acetate beads is very useful and efficient and the immobilized enzyme can be used in all enzymatic, biochemical and industrial applications needed to catalase.
Acknowledgment
This study was supported by Ege University Research Fund.
References
- Aebi H. Methods of Enzymatic Analysis, H. U. Bergmayer. Academic Press Inc., New York 1974; pp. 673–690
- Akgöl S., Dinçkaya E. A novel biosensor for specific determination of hydrogen peroxide: catalase enzyme electrode based on dissolved oxygen probe. Talanta 1999; 48: 363–367
- Çetinus Ş. A., Öztop H. N. Immobilization of catalase into chemically crosslinked chitosan beads. Enzyme and Microbial Technology 2003; 32: 889–894
- Demmano G., Selegny E., Vincent J. C. Experimental procedure for a hydrogen peroxide assay based on the peroxidase-oxidase reaction. European Journal of Biochemistry 1996; 238: 735–739, [CROSSREF]
- Mosbach K. Immobilized enzymes and cells. Methods of Enzymology. Academic Press, San Diego, CA 1988; 137 pp
- Nakoshima K., Wada M., Kuroda N., Akiyama S., Imai K. HPLC determination of hydrogen peroxide with peroxylate chemiluminescence detection. Journal of Liquid Chromatography 1994; 17: 2111–2116
- Stein K., Hain J. U. Catalase biosensor for the determination of peroxide, fluoride and cyanide. Microchimica Acta 1995; 118: 93–101
- Tarhan L., Akertek E. Characterization of immobilized catalases and their application in pasteurization of milk with H2O2. Applied Biochemistry and Biotechnology 1995; 50: 292–297
- Tarhan D., Uslan A. H. Characterization and operational stability of immobilised catalase. Process Biochemistry 1990; 25: 14–18
- Wang F., Schubert F., Rinneberg H. A fluorometric rote assay of hydrogen peroxide using immobilized peroxidase with a fiberoptic detector. Sensors and Actuators 1995; 28: 3–7, [CROSSREF]