Abstract
In this study, catalase enzyme was immobilized by entrapping in alginate beads in the presence of gelatin. In the optimization studies of the bioactive layer immobilized some parameters such as enzyme amount, alginate, gelatin, and crosslinking agent glutaraldehyde amount were determined as 700 U/mL, 2.0%, 18 mg/mL, and 5.0%, respectively. Effects of pH and temperature on the immobilization were also investigated. In the characterization studies of the immobilized enzyme storage and thermal stability experiments were done. The immobilized enzyme was used for the decomposition of hydrogen peroxide in milk samples and also by using a catalase biosensor prepared by the decomposition level of hydrogen peroxide was detected.
Introduction
Catalase is a very common enzyme found in plants, animal cells, and all microorganism in the nature exist in the same species either as a different organ or in the form of izoenzymes in cell organelles. For example, it has been proven that four different variations of genetics exist in human erythrocyte and they are sort of enzymes which have a different structure form to that of human liver (Eventoff, Citation[[1976]]; Ko et al., Citation[[1999]]). Physiological function of catalase has not yet completely been understood but it has been accepted that catalase has a protective function against free radicals composed of oxygen. Hydrogen peroxide is substrate of catalase enzyme and it is especially added into milk in order to prevent microbiological contamination during the process of cold sterilization of milk and also to avoid microbiological growth. As a result of the process all of the hydrogen peroxide is degraded into O2 and H2O by catalase (Amounas et al., Citation[[2003]]). The immobilization of catalase is very important for both economical and yielded usage of the enzyme. In recent years catalase has been used in immobilized form especially in biosensor studies (Akgöl and Dinçkaya Citation[[1999]]; Ertaş et al., Citation[[2000]]; Li et al., Citation[[1994]]; Stein and Hain, Citation[[1995]]).
In this study catalase was immobilized by entrapping in alginate beads. For the optimization of the immobilization procedure some parameters such as effects of content of the biological active layer composition, pH, and temperature were investigated. The immobilized enzyme was used for the decomposition of hydrogen peroxide in milk samples and also by using a catalase biosensor prepared according to Akgöl and Dinçkaya (Citation[[1999]]) the decomposition level of hydrogen peroxide was detected. In this method dissolved oxygen concentration which was related to hydrogen peroxide decomposition determined by using a YSI 57 Model oxygenmeter.
Materials and Methods
Materials
Catalase [E.C 1.11.1.6] from bovine liver, sodium alginate, the 225 bloom calf skin gelatin (Type III), and glutaraldehyde (25%) were obtained from Sigma (St. Louis, USA). Hydrogen peroxide (30%), CaCl2, and all other chemicals used in the experiments were purchased from Merck (Darmstadt, Germany).
Apparatus
Spectrophotometric analyses were carried out with a Jasco V-500 uv/vis spectrophotometer (Japan). In the biosensor experiments a YSI 5739 Model dissolved oxygen (DO) probes based on amperometric consist of Au (cathode), Ag–AgCl (anode), half saturated KCl (electrolyte), a teflon membrane which is selective for oxygen, and YSI 57 Model oxygenmeter (YSI, Yellow Springs, Ohio) and an ultrathermostat (Colora, Germany) were also used.
Immobilization Procedure of Catalase
Catalase was immobilized by using both entrapping and cross-linking methods together. In the entrapping alginate was used as the basic matrix. For the immobilization of catalase 180 mg gelatin was dissolved in 8.0 mL Tris–HCl buffer (pH:7.0, 50 mM) at 35°C throughly using a magnetic stirrer. Alginate (2.0%) was added to the solution and then 2.0 mL of catalase (700 U/mL, in Tris–HCl buffer; pH:7.0, 50 mM) was added to the solution and stirred for 10 min. at 35°C. 5.0 mL of the final mixture was dropped into 25 mL 0.2 M CaCl2 solution (in Tris–HCl buffer, pH:7.0, 50 mM) contained 5.0% glutaraldehyde by using a syringe and stirred for 30 min. After this step, the beads occurred were separated with a decantation procedure and washed with distilled water. The beads were waited in Tris–HCl buffer (pH:7.0, 50 mM) for 30 min and decanted again. After washing the beads with distilled water they were stored in moisted filter paper at 4°C.
Measurement of Free and Immobilized Catalase Activity
Activity mesurement of free and immobilized catalase was made for 10.5 mM hydrogenperoxide at 240 nm by using a uv/vis spectrophotometer according to Aebi (Citation[[1974]]). Activity of catalase immobilized was accepted as µmol/dk g (immobilized beads) or IU/g (immobilized beads). The molar extinction coefficient is 40 cm2/µmol at this wavelength.
Preparation of Catalase Biosensor
Catalase (9324.2 IU/mL) and 225 bloom gelatin (33.3 mg/mL) were mixed at 38°C in distilled water. 200 µL of the mixed solution was spread over dissolved oxygen (DO) probe membrane and allow to dry at 4°C for 1 h. Finally, it was immersed in 2.5% glutaraldehyde in 50 mM phosphate buffer (pH:7.5) for 3 min. The biosensor prepared contained 1650.3 IU/cm2 and 5.9 mg/cm2 gelatin in the bioactive layer.
Results and Discussion
Optimization Studies
In this section of the study effect of pH; temperature, buffer system, and buffer concentration on the free and immobilized catalyzes were investigated.
Effect of pH
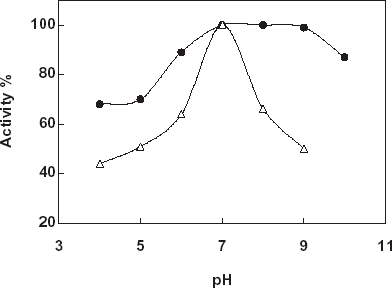
For the determination of the effect of pH on the free and immobilized catalase buffers that is in the pH range 4–10 were used. In the experiments Na-acetate buffer (pH: 4.0, 5.0, and 5.5; 50 mM), Tris–HCl buffer (pH: 7.0 and 8.0; 50 mM), and glycine buffer (pH:9.0 and 10.0; 50 mM) were used. shows the results obtained. According to the figure the highest activity was obtained in pH: 7.0 for free and immobilized catalase. Therefore, the pH profile of the immobilized catalase was much broader with respect to the free enzyme. By using different buffer systems such as Tris–HCl, phosphate and citrate (pH:7.0, 50 mM) the most suitable buffer system for the enzyme activity was determined. From the experiments Tris–HCl buffer system gave the most suitable results. After that, Tris–HCl buffers at varying concentrations (25, 50, 100, and 200 mM) were prepared and tested for the maximum enzyme activity. According to the results for both free and immobilized catalase the maximum activity was taken when 50 mM concentration of the buffer used.
Effect of Temperature
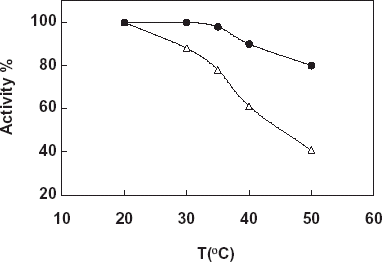
Determination of the effect of temperature on enzyme activity was investigated in Tris–HCl buffer in a temperature range 30–40°C. ().
Figure 2. Effect of temperature on free and immobilized catalase, -Δ-: free catalase, -•-: immobilized catalase (pH 7.0: Tris–HCl buffer, 50 mM).
From the figure it can be said that the optimum temperature for both free and immobilized catalase in the working conditions is found as 35°C. If we consider the optimum temperature of free enzyme given in literatures and enzyme handbooks as 35°C we can say that immobilization procedure didn’t show an effect on the optimum temperature of the enzyme. However, an increase in the temperature stability of the immobilized enzyme was observed. This result can be explained by the occuring of a conformational stability results from the immobilization of enzyme.
Characterization Studies
In order to investigate characterization of the free and immobilized catalase enzyme some parameters such as thermal and pH stability experiments were done.
Thermal Stability of the Free and Immobilized Catalase
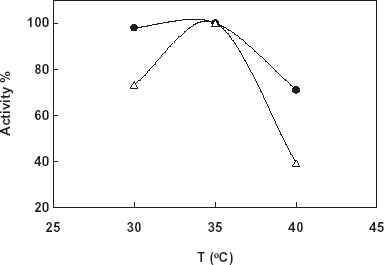
In this study the free and immobilized catalase were incubated at different temperatures (20–50°C) for 18 h. At the end of the time under the optimum conditions the activities of the enzymes were assayed. shows the results obtained. From the figure it can be said that immobilization was gained a thermal stability to the enzyme related to increase its conformational stability by the immobilization. This effect is very clear and increase at higher temperatures from 20°C.
pH Stability of the Free and Immobilized Catalase
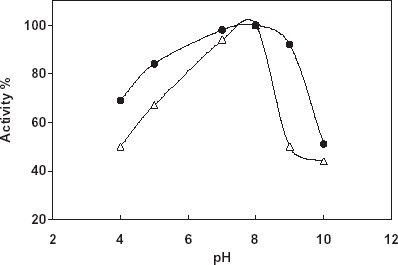
For the determination of pH stability free and immobilized catalase were incubated in buffers that is in the pH range 4–10 for 18 h at 20°C. At the end of the time the activity measurements of the enzymes were made under the optimum assay conditions (). As it is shown from the figure pH stability of the both enzymes is similar. Free catalase gave an optimum at pH:8.0, however, the immobilized catalase gave a much broader pH stability between pH:7.0 and pH:8.0 with respect to the free enzyme. The results obtained can be thought as an effect of micro environment of the enzyme and pH on the enzyme stability.
Determination of Hydrogen Peroxide Decomposition in Milk Samples by using Catalase Biosensor
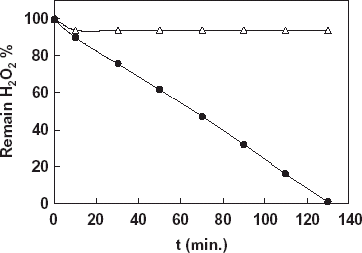
For the determination of the decomposition level of hydrogen peroxide in milk samples by the immobilized catalase, a catalase biosensor and two milk samples contained 0.03 and 0.1% hydrogen peroxide were prepared. In the experiments a YSI Model 57 oxygenmeter and 5739 Model dissolved oxygen probe covered with a oxygen sensitive teflon membrane were used. Biosensor measurements were done in the steady-state condition at 35°C. Before the treatment of the milk samples by the immobilized catalase the hydrogen peroxide concentration of the samples (0.03 and 0.1%) were determined by using the catalase biosensor prepared. The milk samples were treated with the immobilized catalase for 2 h by stirring. During this time, in the beginning and per 20 min samples treated with immobilized catalase were taken from the reaction medium and hydrogen peroxide decomposition level of the milk samples were determined by using catalase biosensor. Addition of the milk samples contained hydrogen peroxide in the reaction cell caused a rapid current increase due to hydrogen peroxide destruction in the enzyme layer on the biosensor (). According to the results given in the figure, it was detected that 10.0% of hydrogen peroxide of sample 1 (0.03%) and 6.25% of hydrogen peroxide of sample 2 (0.1%) decomposed in first 10 min. During from 10 to 120 min there was no change in the decomposition level for sample 2. This results from catalase enzyme denaturation occurred in high concentration of hydrogen peroxide. On the other hand, during the decomposition process there was a periodical increase in the decomposition of hydrogen peroxide for sample 2 and at the end of the time only 0.5% of hydrogen peroxide remained.
Figure 5. Determination of decomposition level of hydrogen peroxide in milk samples by using the catalase biosensor, -•-: Sample 1 contained 0.03% H2O2, -Δ-: Sample 2 contained 0.1% H2O2..
From the all results obtained for catalase enzyme immobilized in alginate beads it can be said that immobilization procedure is very useful and efficient and the immobilized enzyme can be used in all enzymatic, biochemical, and endustrial applications needed to catalase.
Acknowledgment
This study was supported by Ege University Research Fund.
References
- Aebi H. Methods of Enzymatic Analysis, H. U. Bergmayer. Academic Press Inc., New York 1974; pp. 673–690
- Akgöl S., Dinçkaya E. A novel biosensor for specific determination of hydrogen peroxide: catalase enzyme electrode based on dissolved oxygen probe. Talanta 1999; 48: 363–367
- Amounas M., Innocent C., Cosnier S., Seta P. Dismutation of hydrogen peroxide from water medium by catalytic reactive membrane immobilizing peroxidase and catalase by molecular recognition process. Separation Science and Technology 2003; 38: 1296–1305, [CROSSREF]
- Ertaş N., Timur S., Akyilmaz E., Dinçkaya E. Specific determination of hydrogen peroxide with a catalase biosensor based on mercury thin film electrodes. Turk. J. Chem. 2000; 24: 95–99
- Eventoff W. Crystalline bovine liver catalase. Journal of Molecular Biology 1976; 103: 799–801, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ko T. P., Day J., Malkin A., McPherson A. Structure of orthorhombic crystals of beer liver catalase. Acta Crystallography D Biological Crystallography 1999; 55: 1383–1394, [CROSSREF]
- Li J. Z., Zhong Z. J., Li L. A simplified enzyme based fiberoptic sensor for hydrogen-peroxide and oxidase substrates. Talanta 1994; 41: 1999–2002, [CROSSREF]
- Stein K., Hain J. U. Catalase biosensor for the determination of peroxide, fluoride and cyanide. Microchimica Acta 1995; 118: 93–101