Abstract
Bone marrow stromal cells (MSCs) have been shown to differentiate into various lineage cells including neural cells in vitro and in vivo. We therefore examined whether MSCs can differentiate into Schwann cells in injured peripheral nerves. After cultured in vitro, PKH-67-labeled MSCs were injected into the mechanically injured rat sciatic nerves. Three weeks after injection, immunofluorescent examinations were carried out. MSCs had been incorporated around the injured nerves and differentiated into Schwann cells. MSCs had accumulated mainly in the epineurium around the injured nerve. The incorporated cells partially expressed GFAP, S-100, and P75. These results confirmed the possibility that MSCs have the ability to differentiate into Schwann cells, and that injection of MSCs into the injured peripheral nerve would help repair damaged nerve.
Introduction
Bone marrow stromal cells (MSCs) are well known as multipotential stem cells which, under specific inducing conditions, can differentiate into several cells types such as osteoblasts, adipocytes, chondrocytes, and even muscle cells. Although quite different from neuronal lineage, the potential of MSCs to develop into neurons and astrocytes both in vivo (Kopen et al., [Citation1999]) and in vitro (Kim et al., [Citation2002]; Sanchez-Ramos et al., [Citation2000]) has been reported. MSCs have the capacity to differentiate into myelin-forming cells in vivo and to repair demyelinated spinal cord axons (Sasaki et al., [Citation2001]). Mari Dezawa et al. ([Citation2001]) and Pedro Cuevas et al. ([Citation2002]) injected MSCs directly to the nerve injure site and verified its abilities to facilitate nerve regeneration. These findings suggest that MSCs can differentiate into nerve cells under the appropriate in vivo conditions. Based on these research facts, we cultured and enriched the MSCs to the extent of about 107 in vitro and injected them into the artificial mechanically injured peripheral nerves. Our study demonstrates that MSCs can survive and differentiate in vivo, and the differentiated cells take on Schwann cells’ characteristics of peripheral nerve, expressing GFAP, S-100, and P75. MSCs may serve as the possible substitutable cell for artificial nerve conduits.
Materials and Methods
Experiments were conducted in according with the Guide for the Care and Use of Laboratory Animals from the National Academy of Science.
Preparation of MSCs for Transplantation In Vivo
Isolation of adult SD rat MSCs was performed according to the procedures provided by Mari Dezawa et al. ([Citation2001]) and Pedro Cuevas et al. ([Citation2002]). The tibia's and femur's bone marrow was extruded with α-MEM (Gibco) after the rats were killed with overdose pentobarbital. The extrudation fluid is cultured in α-MEM supplemented with 10% fetal bovine serum (Gibco) and 200 mg/mL Kanamycin, incubated at 37°C, humidity 95%, and CO2 5%. Ten nanogram per milliliter leukemia inhibitory factor (LIF, Sigma, US) was added to maintain the MSCs’ differentiation potential (Jiang et al., [Citation2002]). To get rid of the hematic cells, the culture medium was removed twice by virtue of their adherence character to plastic after 48 h. The adhered cells were cultured and passaged to about 6 × 107 in α-MEM supplemented with 10% fetal bovine serum and 10 ng/mL LIF. Digested and collected MSCs were labeled with PKH67 green fluorescent cell linker kit for general cell membrane labeling (Sigma, US) according to the production definitions.
Rats Preparation and Transplantation Procedures
Sqrague-Dawley rats (about 200 g or so; n = 6) were anesthetized with pentobarbital sodium (10 mg/kg body weight), which was injected into the abdominal cavity. One must find the scatic nerve (choose right side, randomly) and damage it to the extent that the nerve fiber is disrupted while the perineurium remains continuous with hemostat pincers. We microinjected the MSCs (0.1 mL in α-MEM, about × 107cells/inject site) into the injured site, and wrapped the exact injured site with an artificial absorbable chitin nerve conduit to get a relative fitting differentiation microenvironment and labeled the injection site, inner diameter 1.5 mm and long 10 mm (patented, national patent administration, China, 2002). As control, 0.1 mL normal saline solution was injected into the left side.
Tissue Sectioning
The animals were sacrificed 3 weeks after injection. The injected nerves were collected and embedded in OCT. Five micrometer continuous frozen sections were made in a cryostat. Sections were fixed with 4% paraformaldehyde immediately.
Antibodies Applied
Primary antibodies used in this study were as follows: rabbit anti-GFAP (Sigma), rabbit anti-S-100 (Sigma), rabbit anti-P75 (Sigma). Second antibodies used in this study were as follows: TRITC-conjugated Affinipure goat anti-rabbit Ab IgG(H + L), (Jackson Immunoresearch Labs, US).
Immunofluorescence Staining
The specimens, which had been fixed with 4% paraformaldehyde, were stained with rabbit Abs (anti-rat-GFAP, anti-rat-P75, and anti-rat-S-100) followed by staining with TRITC-conjugated Affinipure goat anti-rabbit Ab IgG(H + L). The stained specimens were all observed using a fluorescent microscope, after being redyed by DAPI (Olympus XI 70, Japan), and the Leica laser confocal microscopy (Germany).
Results
Incorporation and Distribution of Injected Cells
The patterns of distribution of the grafted cells were similar in all six experiments. Grafted cells were observed mainly at the site of injury, where the normal nerve fiber structure had been destroyed, but there was also green fluorescence in the surrounding area, where the normal nerve fiber structure had been retained. The grafted cells, which expressed green fluorescence, were distributed around the injured nerve sites. (see )
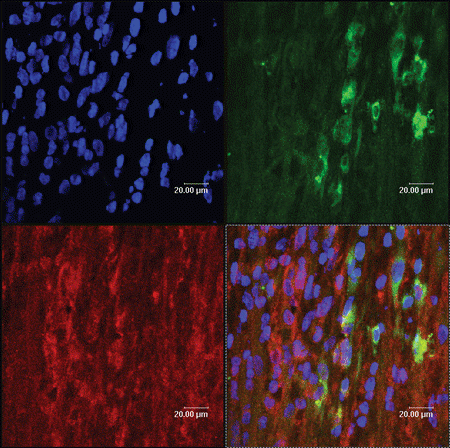
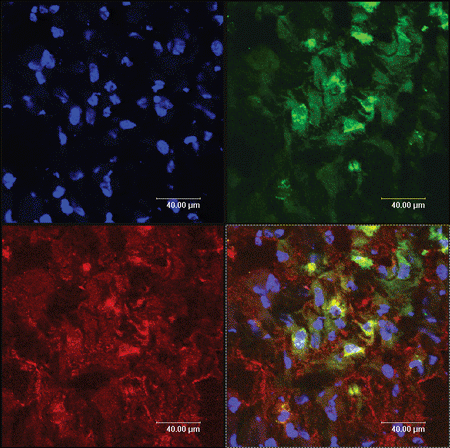
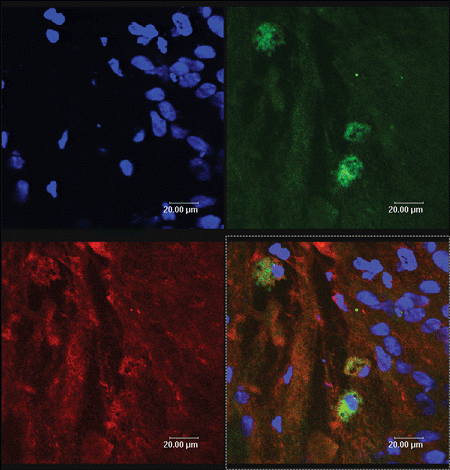
Figure 1. The PKH-67 labeled MSC (green) after injection accumulated around the nerve fiber stem and some came into the nerve fiber stem and took on Schwann specific makers. (Yellow) primary antibody is anti-rat-S-100 and second antibody is TRITC-conjugated Affinipure goat anti-rabbit Ab IgG(H + L). The nerve fibers display positive reactions to anti-rat-S-100 (Red) (frozen sections, Olympus XI 70 fluorencent microscope, Japan).

Immunofluorescence on Sections after Transplantation
The cell-type markers used to confirm Schwann cells differentiation were GFAP, S100, and P75, which were regarded as the maker of peripheral nerve glial cells, namely Schwann cells. The injected cells, which had been labeled with a PKH-67 kit, emitted green fluorescence, while expressions of GFAP, S100, and P75 were detected with red fluorescence. Therefore after immunofluorescence staining, the incorporated MSCs-derived cells expressing GFAP, S100, and P75 should express yellow fluorescence (arrows), while injected cells that do not express the glial-specific markers express green fluorescence. The cells expressing yellow fluorescence are shown in . These data demonstrated that incorporated MSCs had partially differentiated into Schwann cells. Representative data from six independent experiments are shown.
Figure 2. The yellow color sites (arrow) represent these PKH67 labeled MSCs (green) and their expression of GFAP (red).
Discussion
Schwann cells contribute to nerve regeneration by providing regeneration strands, kinds of nerve growth factors and tight junctions to stabilize cell contact with injured nerve cells, as well as gap junctions to facilitate traffic of substances between the cells (Dezawa and Adachi-Usami, [Citation2000]). Although Schwann cells’ function of facilitating nerve regeneration has been confirmed, the Schwann resource and the xenogenic graft immunorejection reaction still remain. It has been reported that MSCs have the ability to differentiate into various lineage cells (Azizi et al., [Citation1998]; Brazelton et al., [Citation2000]; Ferrari et al., [Citation1998]; Krause et al., [Citation2001]; Orlic et al., [Citation2001]). Junko Hori et al. ([Citation2003]) reports that neural progenitor cells lack immunogenicity and can survive in nonimmune-privileged sites. Yuehua Jiang et al. ([Citation2002]) reported that MHC-I and MHC-II were both negative in MSCs. Shengkun et al. ([Citation2003]) reported MSCs did not express MHC-II and T-cell co-stimulatory molecules CD80, CD86. Minoru Tomita et al. ([Citation2002]) reported that PKH67-labeled bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Considering all these facts we observed the biological characteristics of MSCs in nonimmune-privileged site and examined whether they can suvive in vivo and even differentiate into Schwann cells in injured nerves.
The PKH-67-GL cell linker kit uses patented membrane labeling technology to stably incorporate a fluorescent dye with long aliphatic tails (PKH67) into lipid regions of the cell membrane. With reduced cell–cell transfer, PKH67 has been found to be useful both in vitro cell proliferation labeling (Boutonnat et al., [Citation2000]) and in vivo cell tracking applications for a relatively short time (Askenasy et al., [Citation2002]). Labeled by PKH67, MSCs which have differented into Schwann cells, expressed yellow fluorescence overlapped by green and red fluorescence, while those who do not express the glial-specific markers only express green fluorescence.
The artificial nerve conduit may not only act as a kind of shield to maintain the MSCs differentiation microenvironment, but also act as guide for regenerated nerve fiber orientation.
During our cell culture process, we found that without the addition of LIF, the MSCs’ differentiation potential was partially lost and our first experiment was thus a failure. After the LIF addition during the cell culture according to Yuehua Jiang's definition (Jiang et al., [Citation2002]) we got the satisfactory differentiation results.
In this report, our data have shown that MSCs injected into injured nerve can survive and differentiate into Schwann cells in vivo. In the present study, transplanted MSCs expressed GFAP, S100, and P75, which are considered to be Schwann cell-specific markers. Marrow stromal cells are certainly one of the most attractive cells for an application of neural transplantation because they exhibit several important and potentially advantageous features. They are easy to isolate from bone marrow aspiration in the clinic and can readily be expanded under culture conditions for transplantation. Therefore, differentiated MSCs are considered by us to become among the strongest candidates for cell transplantation involving tissues of the nervous system.
Acknowledgments
We thank Professor Micheal Brenner (Associate Professor of Neurobiology with a joint appointment in the Department of Physical Medicine and Rehabilitation, Alabama, USA) for his technical assistance and suggestion.
This research work was supported by Chinese National 863 Project Grant (2002AA205071) and Chinese National Nature Science Grant (30271306).
References
- Askenasy N., Daniel L., Farkasa C. Antigen barriers or available space do not restrict in situ adhesion of hemopoietic cells to bone marrow stroma. Stem Cells 2002; 20: 80–85
- Azizi S. A., Stokes D., Augelli B. J., DiGirolamo C., Prockop D. J. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats. Proc. Natl. Acad. Sci. USA 1998; 95: 3908–3913
- Boutonnat J., Barbier M., Muirhead K., Mousseau M., Grunwald D., Ronot X., Seigneurin D. Response of chemosensitive and chemoresistant leukemic cell lines to drug therapy: simultaneous assessment of proliferation, apoptosis, and necrosis. Cytometry 2000; 15;42(1)50–60
- Brazelton T. R., Rossi F. M., Keshet G. I., Blau H. M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 2000; 290: 1775–1779
- Cuevas P., Carceller F., Dujovny M., Garcia-Gomez I., Cuevas B., Gonzalez-Corrochano R., Diaz-Gonzalez D., Reimers D. Peripheral nerve regeneration by bone marrow stromal cells. Neurological Research 2002; 24: 634–638
- Dezawa M., Adachi-Usami E. Role of Schwann cells in retinal gsnglion cell axon regeneration. Prog. Retin. Eye Res. 2000; 19: 171–204
- Dezawa M., Takahashi I., Esaki M., Takano M., Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. European Journal of Neuroscience 2001; 14: 1771–1776
- Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998; 279: 1528–1530
- Hori J., Ng T. F., Shatos M., Klassen H., Streilein J. W., Young M. J. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells 2003; 21: 405–416
- Jiang Yuehua, Jahagirdar, Balkrishna N., Reinhardt R. Lee, Schwartz, Robert E., Keene C. Dirk, Ortiz-Gonzalez, Xilma R., Reyes Morayma, Lenvik Todd, Lund Troy, Blackstad Mark, Du Jingbo, Aldrich Sara, Lisberg Aaron, Low Walter C., Largaespada David A. Pluripoency of meschymal stem cells derived from adult marrow. Nature 2002; 418: 41–49
- Kim B. J., Seo J. H., Bubien J. K., Oh Y. S. Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. Neuroreport 2002; 13(8)1003–1004
- Kopen G. C., Prockop D. J., Phinney D. G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl. Acad. Sci. USA 1999; 96: 10711–10716
- Krause D. S., Theise N. D., Collector M. I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S. J. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001; 105: 369–377
- Orlic D., Kajstura J., Chimenti S., Bodine D. M., Leri A., Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 2001; 410: 701–705
- Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T., Willing A., Freeman T. B., Saporta S., Janssen W., Patel N., Cooper D. R., Sanberg P. R. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000; 164: 247–256
- Sasaki M., Honmou O., Akiyama Y., Uede T., Hashi K., Kocsis J. D. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia 2001; 35: 26–34
- Shengkun Sun, Zikuan Guo, Xuren Xiao, Bing Liu, Xiaodan Liu, Pei-Hsien Tang, Ning Mao. Isolation of mouse marrowmesenchymal progenitors by a novel and reliable method. Stem Cells 2003; 21: 527–535
- Tomita M., Adachi Y., Yamada H., Takahashi K., Kiuchi K., Oyaizu H., Ikebukuro K., Kaneda H., Matsumura M., Ikehara S. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells 2002; 20: 279–283