Abstract
The membranes preventing tissue overgrowth as well as toxic influence on cells encapsulated within can be obtained modifying the polypropylene membranes by silanization. The influence of the silanization with different siloxanes on membrane transport properties was assessed before and post implantation. No change in cut-off values was observed. All of the modified membranes delayed tissue overgrowth of implant in mouse. Spectroscopic evaluation of the membrane material after 4, 7 days, 2 and 4 months of implantation revealed membrane material stability. We concluded that evaluated membranes with cells encapsulated within may be applied as the systems for delivery of biologically active substances.
Introduction
Transplantation of cells secreting biologically active substances may be a way of treatment in case of deficiency of these factors. Encapsulation of cells in the permiselective membranes is a way to avoid immunological rejection by a host.
We elaborated permiselective membranes for immunoisolation of cells, which exhibit diffusive permeability sufficient for transport of small solutes (like nutrients) and a cut-off allowing one to avoid direct contact of encapsulated cells with host immunocompetent cells as well as with some of the complement proteins indispensable for their activation, e.g., the first element of the complement C1q protein (MW 410 kDa) (the passage of this protein through the elaborated membrane probably can not be possible).
Materials and Methods
Materials
Hollow fibers (HF) of polypropylene K600 PP, Accurel (Akzo-Nobel, Wuppertal, Germany), inner diameter 0.6 mm, wall thickness 0.2 mm; Solvent: n-hexane spectrally pure (MERC); Siloxanes (SERVA): OV1 (100% methylosilan), OV17 (50% methylosilan, 50% phenylosilan); Reagents: creatinine (Sigma, Germany, 99% purity), bovine IgG (WWSS, Poland, 98% purity); physiological saline.
Animals. Mouse CDF1 (BALB/c × DBA/2)F1, body weight about 20 g, on standard diet and water ad libitum were used. The protocol for animal experiments was examined and approved by the local ethical committee.
Methods
Hollow Fibers Silanization
Hollow fibers K600 PP were stored for 15 min in ethanol, then immersed in siloxane solution in n-hexane at concentration 1% (OV1), or 5% (OV17) for 5 min at 20°C and at atmospheric pressure.
Evaluation of Diffusive Permeability
The diffusive permeability assessment is an adequate method for evaluation of membranes for cell immunoisolation, because of the flow conditions in measurement system (described below) adequate to flow conditions in system HF-body fluids environment.
To evaluate the HF diffusive permeability the thermodynamic description of diffusive mass transport (Fick's law) and a 2-compartment model were applied (Granicka et al., [Citation1996]). Briefly, the HF were stored for 30 min in 70% ethanol and then washed in distilled water followed by washing in physiological saline for 15 min each. The HF were filled with tested solute solution in saline, sealed on the both ends and immersed in a beaker with continuously mixed physiological saline. The samples were taken from the beaker at various time periods to evaluate the solute concentration in the direct measurement in the spectrophotometer (Shimadzu 160, Japan), at 235 and 280 nm wave length for creatinine and bovine IgG, respectively.
Spectroscopic Evaluation
Evaluation of spectrum of absorption for red irradiation (FTIR) was performed to evaluate the chemical stability of membrane modification before and after implantation, using a FTS3000 MX (BioRad Excalibur, USA) device. The 16 scans were collected with resolution 4 cm−1.
Experiments In Vivo
It was observed in previous experiments that modification of polypropylene membranes by silanization improves biocompatibility as compared to the original ones (Granicka et al., [Citation2000]). In this study, membranes silanized with two different viscosity and polarity siloxanes were examined in vivo in 14 experiments.
Hollow Fibers of polypropylene K600 silanized OV1 or OV17 were implanted subcutaneously into vetbutal anesthetized mice for: (a) short time: 4 days (n = 3) or 7 days (n = 3). The spectroscopic evaluation of membranes was performed after explantation. (b) 2 months (n = 4). The diffusive permeability evaluation of the membranes after explantation as well as spectroscopic evaluation was performed. (c) 4 months (n = 4). FITR spectrum evaluation and electron microscopy evaluation was performed.
For microscopic evaluation (n = 4) samples of HF were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 h at 4°C. After washing with the buffer the material was postfixed in 1% osmic acid in 0.1 M cacodylate buffer (pH 7.4) for 2 h at 4°C, following dehydration in gradient of ethyl alcohol and propylene oxide. Then the material was embedded in epoxy resin (Agar 100, resin). HF from 4 blocs of embedded material were taken randomly and cut into thin sections. After staining with methylan blue the material was examined in a light microscope (Nikkon E 400).
Results and Discussion
Experiments In Vitro
The diffusive permeability (P) was evaluated for K600 silanized OV1 or OV17, before implantation and after 2 months of implantation into mice. The obtained values of membrane diffusive permeability for creatinine and IgG are presented in .
Figure 1. The diffusive permeability values of silanized OV1 or OV17 membranes before implantation and after explantation.
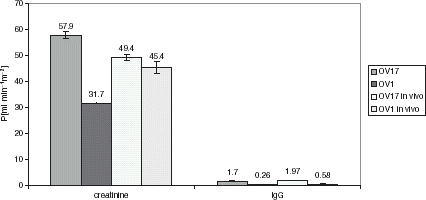
The diffusive cut-off of HF silanized OV1 or OV17 was estimated previously (data not presented), presuming that the HF cut off is equal to the molecular weight value when the ratio of diffusive coefficient in HF (evaluated as described in (Granicka et al., [Citation1996]) and briefly above) to diffusive coefficient in water (DM/DH2O) is equal or lower than 5%.
The diffusive cut off of K600 silanized OV17 or OV1 is, respectively, about 200 and 150 kDa.
Before implantation, the HF silanized with siloxane of higher viscosity (OV1) exhibited lower permeability for both evaluated solutes (and lower cut-off) as compared to HF silanized with lower viscosity siloxan (OV17). No influence of siloxanes of different polarity on HF diffusive transport was observed.
Comparing the P [ml min−1 m−2] value before and post implantation, it was found that the value of P post implantation for OV17 silanized HF decreased for creatinine, from 57.9 ± 1.4 before implantation to 49.4 ± 0.8 post implantation. The value obtained for IgG differ in limits of error from 1.7 ± 0.2 before implantation to 1.9 ± 0.1 post implantation. For OV1 silanized HF the P value increased for creatinine from 31.7 ± 0.2 before implantation to 45.4 ± 2.2 post implantation. The value obtained for IgG differ in limits of error, from 0.26 ± 0.16 before implantation to 0.58 ± 0.31 post implantation.
The changes in permeability value for creatinine before and post in vivo for OV1 and OV17, might be caused by physical changes in membranes during implantation period in vivo, like closing or stretching of pores, and by different tendency to surface deposition of proteins on modified by different siloxanes membranes, because of their different polarity: the K600 silanized OV17 HF are hydrophobic membranes and K600 silikonized OV1 are hydrophilic membranes, with polarity respectively 1.0% and 25.8% (own data).
The chemical changes in membrane structure must be excluded as no changes were observed during FITR evaluation.
The ratio of diffusive coefficient of evaluated membranes (D = Pg, g-wall thickness) to diffusive coefficient in water for tested solutes is presented in .
Figure 2. The ratio of solute diffusive coefficient in membrane to solute diffusive coefficient in water.
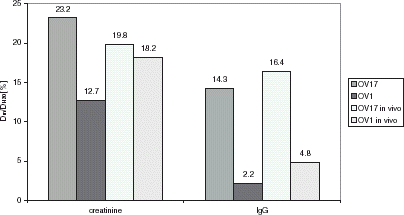
However, the obtained values differ before and post implantation, no change in cut-off values for K600 silanized OV1 or OV1 was observed.
Evaluated, surface modified membranes were permeable for large solutes. In the opinion of many authors (Aebischer et al., [Citation1994]; Lanza et al., [Citation1995a]; Monaco and Maki, [Citation1996]), the HF should be impermeable to immunoglobulins and complement components in order to avoid the immune attack. Nevertheless, in short-time experiments performed with implantation of different cell types encapsulated in polypropylene silanized HFs (cut off 200 kDa), the cells were viable (Granicka et al., [Citation1996], Citation[1999]). Lanza et al. ([Citation1995b]) successfully used alginate, permeable for particles of MW over 600 kD, for encapsulation of pig Langerhans islets, for over 10 weeks.
The parameters of elaborated membranes indicate that they should allow or to avoid direct contact between cytotoxic T-lymphocytes and transplanted cells, and the passage of the larger complement components necessary for its activation and cell membrane attack complex (MAC) formation, which should be sufficient for immunoprotection of encapsulated cells, in spite of the diffusion of encapsulated cells’ elements, evoking the humoral response.
It was also found that there were no differences between K600 silanized OV1 or OV17 before and post explantation after 2, 4 days, and 4 month of implantation, in FTIR spectrum, which leads to the conclusion that applied membranes ascertain stable molecular structure. The exemplary picture of FTIR spectrum for HF K600 OV1, before and post 4 month implantation, is presented in A, B, and C.
Evaluation of Tissue Overgrowth
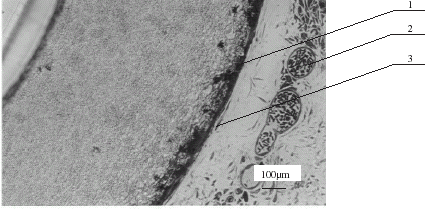
After 2 or 4 months' implantation of membrane silanized OV1 or OV17, the external surface of HFs was surrounded by the thin layer of multinuclear cells and by the thin layer of fibroblasts (). There was no inflammatory reaction with macrophages or limphocyte infiltration behind the layer of fibroblasts, which separate the membrane from further host tissue. We observed that the modification of HFs improves their biocompatibility. In experiments performed previously (Granicka et al., [Citation2003]), it was observed that K600 original, implanted subcutaneously in mice evoked inflamatory reaction on the surface of the HFs after 105 days of implantation.
Figure 4. The fragment of the HF wall, modified with OV1 wall after 2 months of subcutaneous implantation into mouse. The external surface of HF was surrounded by the thin layer of multinuclear cells and a thin layer of fibroblasts. At some distance from the surface of HF are capillary blood vessels containing numerous erythrocytes. 1—multinuclear cells, 2—capillary blood vessels containing numerous erythrocytes, 3—fibroblast.
Conclusions
Membranes undergoing silanization by different applied siloxanes do not induce massive tissue overgrowth and scar formation after implantation in vivo up to four months.
Applied membranes ascertain stable diffusive cut-off and the stable molecular structure.
We observed that modified HF membranes are not undergoing massive connective tissue overgrowth as compared to unmodified membranes. They are characterized by stable physical and chemical parameters and with biological material enclosed within, may serve as a system for production of biologically active substances in applications in vivo.
References
- Aebischer P., Buchser B., Joseph J. M., Favre J., Tribolet N., Lysaght M., Rudnick S., Goddard M. Transplantation in humans of encapsulated xenogenic cells without immunosuppression. Transplantation 1994; 58(11)1275–1277
- Granicka L., Kawiak J., Głowacka E., Weryński A. Encapsulation of OKT3 cells in hollow fibers. ASAIO Journal 1996; 42(2)M863–M866
- Granicka L. H., Migaj M., Zawitkowska T., Woźniewicz B., Tołłoczko T., Weryński A., Kawiak J. Evaluation of human parathyroid cells functioning encapsulated in polypropylene hollow fibers. Cell Transplantation 1999; 8(2)165
- Granicka L. H., Migaj M., Woźniewicz B., Zawitkowska T., Tołłoczko T., Weryński A., Kawiak J. Encapsulation of parathyroid cells in hollow fibers: a preliminary report. Folia Histochemica et Cytobiologica 2000; 38(3)129–131
- Granicka L. H., Kawiak J., Snochowski M., Wójcicki J. M., Sabalińska S., Weryński A. Polypropylene hollow fiber for cells isolation. Methods for evaluation of diffusive transport and quality of cells encapsulation. Artificial Cells, Blood Substitutes and Immobilization Biotechnology 2003; 31(3)251–264
- Lanza R., Ecker D., Kuhtreiber W., Staruk J. E., Marsch J., Chick W. L. A simple method for transplanting discordant islets into rats using alginate gel spheres. BioHybrid Technologies 1995a; 59: 1485–1487
- Lanza R., Kuhtreiber W., Ecker D., Staruk J. E., Chick W. L. Xenotransplantation of porcine and bovine islets without immunosuppresion using uncoated alginate microspheres. Transplantation 1995b; 59(10)1377–1384
- Monaco A. P., Maki T. Islet transplantation using immunoexclusion methods. Transplant. Proc. 1996; 28(4)2042–2045


