Abstract
Background. Blood substitutes are being developed using molecular solutions of modified free hemoglobin; however, anaphylactic reactions, severe renal toxicity, and hypertension have been reported in experimental models and human beings. Hypertension remains as an obstacle to the clinical use of most blood substitutes. Several investigators suggest that this effect is due to the interaction between nitric oxide and hemoglobin into the endothelial cells; hence, prevention of hemoglobin extravasation would avoid vasoconstriction. The forms of hemoglobin likely to prevent extravasation include polymerized and encapsulated Hb. Another alternative and significantly less expensive approach is the hydroxyethyl starch Hb-polymer. The aim of the present study was to compare the effect of hydroxyethyl-starch-hemoglobin with that of stroma-free hemoglobin on the in vitro contractile activity of aortic rings isolated from adult male rats. Methods. The hemoglobin-based oxygen carrier was made using stroma-free hemoglobin prepared from outdated human red cells and conjugated with 10% hydroxyethyl starch 200–260 MW. The experiments were made in thoracic segments of the aortic rings incubated with hemoglobin, starch-hemoglobin or Ringer Krebs-Bicarbonate solution (RKB) during 30 min. Smooth muscle contraction with phenylephrine and subsequent inhibition of contraction with carbachol were performed before and after incubation with hemoglobin, starch-hemoglobin, or vehicle. Results. Incubation with hemoglobin and starch-hemoglobin significantly increased the contractile response to phenylephrine of aortic rings compared with RKB solution. The maximal response to carbachol was significantly decreased in the aortic rings incubated with either hemoglobin or starch-hemoglobin in comparison with the RKB-incubated tissues. There were no differences between the aortic rings incubated with either hemoglobin, or starch-hemoglobin. Conclusions. These results show that there are no differences between the effects of stroma-free hemoglobin and starch-hemoglobin on the in vitro contractile activity of aortic rings isolated from adult male rats. Our findings do not support the hypothesis that an increase in the size of the hemoglobin molecule prevents hemoglobin extravasation, and the consequent vasoconstriction due to the scavenging of nitric oxide by stroma free hemoglobin in the cellular space between endothelium and smooth muscle.
Introduction
Substitution of red blood cells with soluble hemoglobin (Hb)-based oxygen is a challenge of modern medicine. Interest in the use of hemoglobin as an oxygen carrier was first reported in the literature in 1934 when totally exsanguinated sheep were transfused with a solution of bovine Hb (Amberson et al., [Citation1934]).
In the late (1940s), Amberson et al. carried out unsuccessful attempts at human transfusion with Hb solution (Amberson et al., [Citation1949]). Anaphylactic reactions (Amberson et al., [Citation1949]), severe renal toxicity (Brandt et al., [Citation1951]), and hypertension (Hess and Reiss, [Citation1996]) have been reported as side-effects of the use of Hb solution. Anaphylactic reactions and renal damage due to stroma-free hemoglobin (SFH) have been solved, but hypertension remains as an obstacle to the clinical use of most blood substitutes.
In animal studies, Schultz et al. have observed that the administration of SFH increases both diastolic and systolic pressures, peaking 15–30 min after administration and returning to baseline levels after two hours (Shultz et al., [Citation1993]).
The mechanism involved in the hypertensive effect of SFH is not completely understood and several explanations have been proposed. Most investigators suggest that this effect is due to the interaction between nitric oxide (NO) and hemoglobin; unlike Hb in the red blood cells, soluble Hb may be taken up by endothelial cells or enters the space between these and smooth muscle and reacts with NO to form met-Hb and NO-Hb (Sanders et al., [Citation1996]).
Nitric oxide, also referred to as endothelial-derived relaxing factor, is a potent endothelial vasorelaxant that inhibits conversion of pro-endothelin into the vasoconstrictor endothelin (Palmer et al., [Citation1987]). On these grounds, prevention of Hb extravasation would avoid vasoconstriction.
The forms of hemoglobin likely to prevent extravasation include polymerized and encapsulated Hb. This type of modification should hinder extravasation, allowing nitric oxide to exert its physiological vasorelaxant role.
In vitro studies on Hb-induced vasoactivity have shown that changes of the Hb molecule cause hypertension that seems qualitatively and quantitatively dependent on the type of modification performed (Rohlfs et al., [Citation1998]). Conversely, red cell and cellular liposome-encapsulated Hb do not cause either vasoconstriction or hypertension (Rudolph et al., [Citation1997]; Sakai et al., [Citation1999]).
The analysis of the literature on the different Hb molecules modified and commercially developed suggests an inverse relationship between Hb molecular size and the extent of the pressor response, an effect that could be related to the dynamics of NO/Hb interactions (Bassange et al., [Citation1987]).
Conjugation of Hb to an inert material has been proposed as a means to increase molecular size, an effect that has been achieved using polyethylene glycol yielding a hemoglobin-based oxygen carrier (HBOC) with reduced vasoactivity (Conover et al., [Citation1996]; Nakai et al., [Citation1998]). An alternative to this approach is the hydroxyethyl starch Hb-polymer, which may be significantly less expensive to produce; however, its potential role in promoting vasoactivity has not been explored.
The aim of the present study was to compare the effect of hydroxyethyl-starch-hemoglobin with that of stroma-free hemoglobin on the in vitro contractile activity of aortic rings isolated from adult male rats.
Methods
Hemoglobin Production and Purification
Stroma-free hemoglobin (SFH) was prepared from outdated human red cells obtained from the blood bank of the Cardiology Hospital, National Medical Center, Mexican Social Security Institute.
Red blood cells (RBCs) were separated from whole blood by the DeVenuto modified method (De Venuto et al., [Citation1977]). RBCs were washed three times with saline solution (0.9%) and hemolized by stirring in 1.5 vol of distilled water during 30 min at 4°C. The hemolyzed cells were then centrifuged at 4000 × g/30 min at 4°C to yield a hemoglobin solution free of visible particulate matter. This solution was filtered through surgical gauze, and mixed with 100 g of DEAE-52 cellulose (Cheung et al., [Citation1984]) in PBS, pH 7.5 for one hour. The SFH obtained was filtered through a sterile 0.22 µm Millipore filter.
The purity of the product was verified by passing a mixture of SFH and N-acetylcysteine (1:1 molar equivalents) through a Sephadex column C-300, and by analyzing the absorbance curves between 540–573 nm (spectrophotometer Beckman DU) from the fourteen fraction of the Sephadex column.
Total Hb content and percentage of HbO2, met-Hb, and other parameters were evaluated with a IL282 Co-Oximeter (Instrument Laboratories, Lexington, Mass.) and are shown on . The Hb was filtered in 40 µm filter, and was stored in sealed containers in a nitrogen atmosphere.
Table 1. Characteristics of the stroma-free and starch hemoglobin preparations used in the study
Starch-hemoglobin Preparation
The hemoglobin-based oxygen carrier was made using 10% hydroxyethyl starch 200,000–260,000 MW (308 mOsm/L) from Fresenius, Bad Homburg, Germany (Sommermeyer et al., [Citation1987]).
The covalent reaction was initiated by adding 0.3 g of cyanogen chloride to 5.0 g of hydroxyethyl starch at 10°C under a nitrogen atmosphere for about 90 min, during which time the pH was maintained in 5.0 with 0.1 M NaOH (Cerny et al., [Citation1982]). This solution produced the “activated starch” and was allowed to stand overnight at 4°C. Twenty-five grams of the SFH solution were transferred to 100 mL of “activated starch” in nitrogen atmosphere for 90 min at room temperature. To regulate the osmolarity the solution was filtered in 40 µm dialysis membrane with human dialysis solution (5.7 g NaCl, 3 g de NaHCO3, 0.28 g de CaCl2, 0.30 g de KCl y 0.15 g de MgCl2 6H2O,/1L) at 4°C overnight. The purity of the starch-hemoglobin solution was verified as mentioned ().
Contractile Activity Assays
Male Sprague-Dawley rats (250–350 g body weight) were killed by cervical dislocation. The thorax was opened by a midline incision, the heart and lungs were put aside, and the aorta was carefully excised. Thoracic segments of the aorta were placed in Ringer Krebs-Bicarbonate solution (RKB) composition (mM): NaCl, 118; KCl, 4.8; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 24; glucose, 11. The thoracic aorta was cleaned of connective tissue and blood, and transversal rings 4–5 mm long were obtained.
Each aortic ring was placed in a 5.0 mL bath with RKB solution, pH 7.4, at 37°C and continuously gassed with 95% O2–5% CO2. The rings were fixed by stainless steel hooks to the bottom of the tissue bath and to a tension transducer (Grass, FT03) connected to a polygraph (Grass, model 7B). Tissues were stabilized under a 2 g tension during a 60 min period. The RKB solution was washed out every 10 min. After the stabilization period the aortic rings were stimulated with KCl 60 mM prepared from RKB by equimolar substitution of KCl by NaCl. All tissues showing two consecutive similar responses to high KCl were included in the study.
Tissues were contracted with phenylephrine (10−6 M), and when a plateau was reached tissues were relaxed with cumulative doses of carbachol (10−8 to 10−5 M), in such a fashion that every 10 min the consecutive dose was added to the bath, without washing out the previous one. At the end of the carbachol curve, the solution in the bath was washed out and replaced with fresh RKB, and the aortic rings were incubated with hemoglobin (1.8 µM), starch-hemoglobin (1.8 µM) or RKB solution during 30 min. The schedule of phenylephrine and carbachol administration was repeated after the incubation time.
Tissue viability was verified by high KCL stimulation at the end of the experiments.
The dose of 1.8 µM was selected to perform experiments on the basis of previous concentration-response curves post incubation with hemoglobin (0.3, 1.0, 1.8, 3.2, and 10 µM). Four solutions of hemoglobin and four of starch-hemoglobin were prepared.
An acceptable level of methemoglobin content (or oxidation state, <5%) of the solutions was verified. Three experiments were performed with each solution.
Data Analysis
The amplitude of responses was recorded. Relaxation was expressed as percentage of the inhibition of the maximal contractile response (phenylephrine 10−6 M). All results are expressed as mean of 10–12 experiments ±S.E.M. Each n was obtained from a different animal. The maximal response (Rmax) and the effective concentration 50 (EC50) were calculated with the Sigma Plot 4.01 software. One-way analysis of variance and Bonferroni multiple comparison tests were used to evaluate statistical differences. P < 0.05 was considered significant.
Results
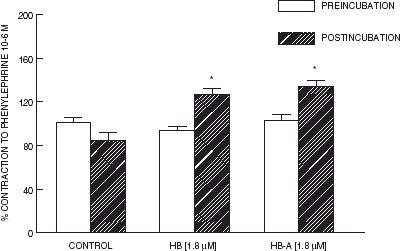
The contraction to phenylephrine () and the carbachol-induced relaxation (data not shown), performed prior to incubation did not show any significant difference between groups. Incubation with hemoglobin and starch-hemoglobin increased the contractile response to phenylephrine of aortic rings, as compared with preincubation response (P < 0.01). Whereas aortic rings incubated with RKB solution seemed to display a minor contractile response to phenylephrine after incubation, however, this difference was not significant ().
Figure 2. Contraction induced by phenylephrine (10−6 M) in rings of aorta isolated from adult male rats. Bars represent mean (n = 10–12) and “T” lines represent S.E.M. *P < 0.01 as compared with preincubation value.
The maximal response to carbachol was minor in the aortic rings incubated with either hemoglobin or starch-hemoglobin in comparison with the RKB-incubated tissues (P < 0.01; ).There were no differences between the aortic rings incubated with either hemoglobin or starch-hemoglobin (). The EC50 of the relaxing response to carbachol was similar in all groups ().
Figure 3. Concentration response curves to carbachol of rings of aorta isolated from adult male rats. Lines represent mean (n = 10–12) and “T” lines represent S.E.M.
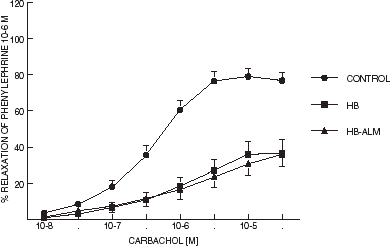
Table 2. Relaxation response to carbachol of aortic rings after precontraction with phenylephrine 10−6 M
Discussion
The results of the present study show that there are no differences between the effects of stroma-free hemoglobin and starch-hemoglobin on the in vitro contractile activity of aortic rings isolated from adult male rats. Therefore, our findings do not support the hypothesis that an increase in the size of the hemoglobin molecule prevents hemoglobin extravasation and thus the vasoconstriction due to the scavenging of nitric oxide by stroma-free hemoglobin in the cellular space between the endothelium and smooth muscle.
In support of this theory, and in contrast with the present findings, studies in a Langendorff perfusion model of rat hearts have shown that polyethylene-glycol-hemoglobin had smaller vasoconstrictive effects than unmodified hemoglobin (Nakai et al., [Citation1998]). The molecular weight of the polyethylene-glycol preparation is about 90,000 Da, whereas that of the starch-hemoglobin used in the present study was 200,000–260,000 Da.
On the basis of the molecular size hypothesis, it would be expected that starch-hemoglobin had a smaller vasoconstrictive effect than both polyethylene-glycol-hemoglobin or stroma-free hemoglobin. Studies with reactive groups of nonheme Hb sites that may also interact with NO have shown that the contribution of these nonheme sites to overall Hb-induced vasoactivity appears to be negligible when the high-affinity heme sites are available for NO interaction (Jia et al., [Citation1996]; Stamler et al., [Citation1997]). These findings suggest that in the starch-hemoglobin molecule assayed in the present study, these high affinity heme sites may still be available for interaction with nitric oxide, which would explain the similarity in vasoactivity between starch-hemoglobin and stroma-free hemoglobin.
Abassi et al. showed that polymeric cross-linked hemoglobin, with molecular size >360 KDa, increased blood pressure in a lesser extent than native hemoglobin in a euvolemic rat model (Abassi et al., [Citation1997]). To explain their findings the authors proposed that:
Polymerization of hemoglobin may not entirely eliminate its propensity to reduce the steady-state flux of nitric oxide toward smooth muscle cells.
Nitric oxide binding to hemoglobin in the lumen will create a diffusion gradient that can theoretically divert more endothelial nitric oxide toward the lumen, where it plays only a partial role in the regulation of vascular tone.
The reduction of local nitric oxide produced by its binding to cell-free hemoglobin can also increase endothelial permeability, reducing the selectivity for differently sized hemoglobins, blunting the effect of size of the molecular species.
This analysis shows the complexity of the processes involved in the vascular tone regulation, since stroma-free and starch-hemoglobin displayed similar effects on the carbachol-induced relaxation in aortic rings.
Nitric oxide binding to ferrous deoxyhemoglobin has been proposed to be the main mechanism for NO depletion. Deoxyhemoglobin has an extremely high affinity for nitric oxide and although its concentration relative to HbO2 may be low, the absolute amount may be still sufficient to bind the nitric oxide produced by endothelial cells (Vandergriff and Winslow, [Citation1995]). Kosaka et al. have shown that stimulation of NO production in endothelial cells by cytokines produces significant amounts of Hb-nitric oxide in vivo (Kosaka et al., [Citation1994]).
Binding to methemoglobin (Hb+) and oxidation/reduction cycles have also been proposed as causes of nitric oxide consumption, although the affinity of nitric oxide for Hb+ is too low to be significant at physiological concentrations (Alayash et al., [Citation1994]).
In the present study, the mean concentration of methemoglobin (1.4 and 4.5 for stroma-free and starch-hemoglobin, respectively) might participate in the nitric oxide scavenging. On the other hand, Rohlfs et al. showed an inverse relationship between the affinity of nitric oxide for hemoglobin and the hypertensive response of conscious rats after 50% hemodilution of intravascular volume, with several modified hemoglobin molecules (Rohlfs et al., [Citation1998]). These findings suggest that the mechanisms participating in the hypertensive response of stroma-free or modified hemoglobin are not completely understood.
Another factor that should be considered is the viscosity of the hemoglobin solution. Tsai et al. have shown that normal levels of tissue perfusion could be obtained during extreme hemodilution when plasma viscosity is increased by the addition of high molecular weight dextran to the blood volume replacement fluid, an effect that could not be obtained with a lower viscosity solution (Tsai et al., [Citation1998]).
Shear stress is a direct function of viscosity. Studies of large conduit arteries have shown a direct relation between the viscosity of the perfusion solution or blood and vessel diameter in various experimental conditions, suggesting that an increase in shear stress, at the lumen surface of the vessel, is the stimulus needed for the enhanced production of the factor(s) eliciting vasodilation (Melkumyants et al., [Citation1989]; Tesfamariam and Halpern, [Citation1987]).
Analysis of the relationships between flow, viscosity, and shear stress in cremaster muscle arterioles from rats has shown that increases in shear stress due to the increases in viscosity of the perfusion fluid resulted in arteriolar dilation similar to that caused by increases in flow velocity, that this dilation was endothelium-dependant, and that it may be mediated by prostaglandins or nitric oxide (Frangos et al., [Citation1996]; Koller et al., [Citation1993]). Thus viscosity, flow, shear stress, and the production of vasodilators are closely related and should be considered in the design of hemoglobin-based oxygen carriers (Intaglietta, [Citation1999]).
In the present study, flow and the consequent shear stress are absent, therefore the stimulus required to induce endothelial production of nitric oxide is not present, which would result in a poor relaxation of the aortic rings. The present study is basically an experimental investigation, therefore it is difficult to draw clinically relevant conclusions, and more studies are needed to clarify the phenomena leading to the hypertensive effect of hemoglobin-based oxygen carriers.
Several questions remain unsolved. May the scavenging of nitric oxide by starch-hemoglobin be compensated by an increase of viscosity of the oxygen carrier? Do hemoglobin-based oxygen carrying blood substitutes have adequate viscosity to maintain endothelial shear stress necessary to stimulate the production of nitric oxide? In this context, new formulations of hemoglobin-based oxygen carriers that enhance viscosity by increasing either the molecular weight or molecule size may be devised by substituting valine and leucine residues by tryptophan in the heme groups of hemoglobin to avoid nitric oxide scavenging without modifying oxygen affinity (De Witt et al., [Citation1997]).
References
- Abassi Z., Kotob S., Pieruzzi F., Abouassali M., Keiser H. R., Fratantoni J. C. Effects of polymerization on the hypertensive action of diaspirin cross-linked hemoglobin in rats. J. Lab. Clin. Med. 1997; 129: 603–610
- Alayash A. I., Brokner Ryan R. E., Frantantoni J. C., Cashon R. E. Redox reactivity of modified hemoglobins with hydrogen peroxide and nitric oxide: toxicological implications. Artif. Cell Blood Substit. Immobil. Biotechnol. 1994; 22: 373–386
- Amberson W. R., Flexner J., Steggerda F. R., Mulder A. G. On the use of Ringer-Locke solution containing Hb as a substitute for normal blood in mammals. J. Cell Comp. Physiol. 1934; 5: 359–382
- Amberson W. R., Jennings J. J., Rhode C. M. Clinical experience with hemoglobin-saline solutions. J. Appl. Physiol. 1949; 1: 469–489
- Bassange E., Busse R., Pohl U. Albumin release and asymmetrical response of the rabbit arterial wall to endothelium-derived relaxing factor. Circ. Res. 1987; 61: 1168–1173
- Brandt J. L., Frank N. R., Lichtman H. The effects of Hb solutions on renal function in man. Blood 1951; 6: 1152–1158
- Cerny L. C., Stasiw D. M., Cerny E. L. A hydroxyethyl starch-hemoglobin polymer as a blood substitute. Clin. Hemorreol. 1982; 2: 355–365
- Cheung L. C., Storm C. B., Gabriel B. W., Anderson W. A. The preparation of stroma-free hemoglobin by selective DEAE-cellulose absorption. Anal. Biochem. 1984; 137: 481–484
- Conover C. D., Lejeune L., Shum K., Shorr R. G. L. The influence of polyethylene glycol conjugation on bovine hemoglobin's intrinsic effect on the gastrointestinal system of the rat. Life Sci. 1996; 59: 1861–1869
- De Venuto F., Zuck T. F., Zegna A. I., Maj M. Characteristics of stroma-free hemoglobin prepared by crystallization. J. Lab. Clin. Med. 1977; 89: 509–516
- De Witt C., Schäfer C., von Bismark P., Pohl U. Elevation of plasma viscosity induces sustained NO-mediated dilatation in the hamster microcirculation in vivo. Pflügers Arch. 1997; 434: 354–361
- Frangos J. A., Huang T. Y., Clark C. B. Steady shear and step changes in shear stimulate endothelium via independent mechanism-superposition of transient and sustained nitric oxide production. Biochem. Biophys. Res. Commun. 1996; 224: 660–665
- Hess J. R., Reiss R. F. Resuscitation and the limited utility of the present generation of blood substitutes. Transfus. Med. Rev. 1996; 10: 276–285
- Intaglietta M. Microcirculatory basis for the design of artificial blood. Microcirculation 1999; 6: 247–258
- Jia L., Bonaventura C., Bonaventura J., Stamler J. S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 1996; 380: 221–226
- Koller A., Sun D., Kaley G. Role of shear stress and endothelial prostaglandins in flow-and viscosity-induced dilation of arterioles in vitro. Cir. Res. 1993; 72: 1276–1284
- Kosaka H., Sawai Y., Sakaguchi H., Kumura E., Harada N., Watanabe M., Shiga T. ESR spectral transition by arteriovenous cycle in nitric oxide hemoglobin of cytokine-treated rats. Am. J. Physiol. 1994; 266: C1400–C1405
- Melkumyants A. M., Balashov S. A., Khayutin V. M. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc. Res. 1989; 23: 741–747
- Nakai K., Sakuma I., Kitabatake A. Vascular activities of hemoglobin-based oxygen carriers. Blood Substitutes Present and Future Perspectives, E. Tushida. Elsevier Science. 1998; pp. 251–264
- Palmer R. M. J., Ferrige A. G., Moncada S. Nitric oxide release accounts the biological activity of endothelium-derived relaxing factor. Nature 1987; 327: 524–526
- Rohlfs R. J., Bruner E., Chiu A. et al. Arterial blood pressure response to cell-free hemoglobin solution and the reaction with nitric oxide. J. Biol. Chem. 1998; 273: 12128–12134
- Rudolph A. S., Sulpizio A., Hieble P. et al. Liposome encapsulation attenuates hemoglobin-induced vasoconstriction in rabbit arterial segment. J. Appl. Physiol. 1997; 82: 1826–1835
- Sakai H., Tsai A. G., Rohlfs R. J., Hara H. Microvascular responses to hemodilution with Hb vesicles as red blood cell substitutes: influence of O2 affinity. Am. J. Physiol. 1999; 276: H553–H562
- Sanders K. E., Ackers G., Sligor S. Engineering and design of blood substitutes. Curr. Opin. Struct. Biol. 1996; 6: 534–540
- Shultz S. C., Grady B., Cole F. A role for endothelin and nitric oxide in the pressor response to diaspirin cross-linked hemoglobin. J. Lab. Clin. Med. 1993; 122: 301–308
- Sommermeyer K., Schmidt F., Weidler B. Klinisch verwendete hydroxyethylstärke-mische charakterisierung. Krankenhauspharmazie, 1987; 271
- Stamler J. S., Jia L., Eu J. P. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 1997; 276: 2034–2037
- Tesfamariam B., Halpern W. Modulation of adrenergic responses in pressurized resistance arteries by flow. Am. J. Physiol. 253 (Heart Circ. Physiol. 22): 1987; H1112–H1119
- Tsai A. G., Friesenecker B., McCarthy M., Sakai H., Intaglietta M. Plasma viscosity regulates capillary perfusion during extreme hemodilution in hamster skin fold model. Am. J. Physiol. 1998; 275: H2170–H2180
- Vandergriff K. D., Winslow R. M. A theoretical analysis of oxygen transport: a new strategy for the design of hemoglobin-based red cell substitutes. Blood Substitutes. Physiological Basis of Efficacy, R. M. Winslow, K. D. Vardergriff, M. Intaglietta. Birkhauser, Boston 1995; pp. 143–154