Abstract
We planted the Lomustine (CCNU) controlled release films into normal mice, and after a period of time observed the effects of the films on the blood cells and the brain nerve cells of the mice. Compared with the traditional administration (P. O.), the results indicated that the planted CCNU controlled release film had less effects on the blood cells, and caused less harm to the brain nerve cells. So the conclusion was that the planted CCNU controlled release film had no significant acute arrest of bone marrow and neural toxicity.
Introduction
Almost all anti-neoplastic drugs inevitably have toxic and side effects on normal cells, when they killed the cancer cells effectively. The cancer patients were already very weak, in pain and with low immunity suffering from the malignancy, so the toxic and side effects induced by the anti-neoplastic drugs indeed quicken the death of patients. Furthermore, just for the intolerability to the toxic and side effects induced by the anti-neoplastic drugs, the disease was delayed without the chemical therapy (Han, [Citation1998]).
There are the following toxic and side effects of anti-neoplastic drugs: (1) arrest of bone marrow, significant decline of white cells (WBC) and platelets (PLT), infection and hemorrhage, accidental decline of red cells (RBC), and hemoprotein (HGB); (2) digestive system reaction, such as nausea, vomitting, diarrhea, and loss of appetite; (3) oral mucous reaction, such as mouth inflammation, pharyngitis, and mouth ulcer; (4) hair loss; (5) accidental neural system toxicity, such as peripheral neuritis. Moreover, some anti-neoplastic drugs have a certain extent effects on immunity, such as the restrain of adrenocortical function.
Before seeing about the curative effects of an anti-neoplastic drug, we should observe its acute, chronic toxicity in animals and humans. The above-mentioned CCNU controlled release films were prepared with Lomustine—a conventional medicine for glioma, and carrier biomaterial—collegan (Sun et al., [Citation2000]). The primary objective of preparing the planted film and changing the administration is to alleviate the toxic reaction, besides increasing the local drug concentration. Therefore the study of toxic reactions is the crucial part.
Materials
CCNU controlled release film, disinfected by radialization; CCNU capsules, Shanghai No. 12 Pharmaceutical Factory; Sodium pentobarbital, Shanghai Medical Reagent Corporation; 2% glutaraldehyde fixative solution, dissolved with 0.1 mol/L Sodium cacodylate solution (pH 7.2); 10% osmium tetroxide, dissolved with 0.1 mol/L Sodium cacodylate solution (pH 7.2); resin, 2% uranium acetate, dissolved with distilled water; plumbum citrate, Shanghai Chemical Reagent Factory, AR; PBS solution (pH = 7.4, 0.04 mol/L); H-800 electronic microscope, Hitachi Corporation, Japan; SYSMEXKX-2 Automatic haemacytometer, East-Asian Medical Electronic Equipment Corporation.
Methods and Results
The mice (weight 18–20 g, half male and half female) were separated into 6 groups (12 mice in every group) randomly; the control group (the collegan film without CCNU), the CCNU capsule group (800 µg/week, P.O.), and CCNU controlled release film group (CCNU 150, 300, 600, 1200 µg/mice). The mice were anaesthetized with sodium pentobarbital, fastened on their back. We incised the mice skin at the left axilla, and put the film into the tissue, then sutured the skin, and observed the survival ratio. Fourteen days later, we put the blood taken out from the mice eyes into tubes with anticoagulant, and determined the value of PLT, RBC, WBC, and HGB of the blood; took out the whole brain, fixed the brain tissue with 10% formalin, then sliced up the pallium and observed the damage of the nerve cells.
At the 14th day after the operation, we found all mice were alive, and the results of haematological index are listed in ; compared with the CCNU 800 µg P.O. group, the CCNU 150, 300, 600 µg film goups had significant increase of WBC and HGB value, the CCNU 150, 300 µg film goups had significant increase of PLT value, indicating that the planted controlled release film of CCNU 150, 300, 600 µg had less haematological toxicity, and less arrest of bone marrow than traditional administration.
Table 1. Effects of CCNU film on blood corpuscles of mice (X ± SD)
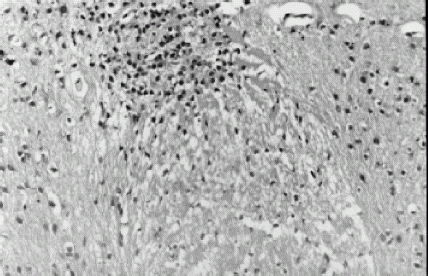
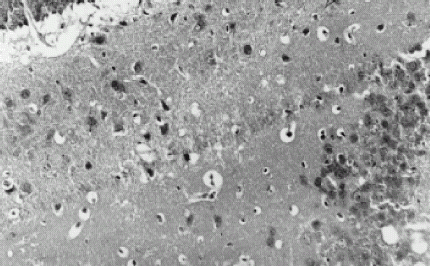
Pathologic examinations of the nerve cells were taken through an electronic telescope. There were no significant abnormities in control group, indicating that the collegan film without CCNU had no toxic effects on the nerve cells of mice; there were some old inteneration regions and increased colloid cells at the pallium in very few samples (2/10) of the CCNU 150, 300 µg film groups, and there were some inteneration regions and the disappearance of the nerve cell nucleus in a few samples (3/10) of the CCNU 600, 1200 µg film groups, indicating that the CCNU film had slight toxic effects on the nerve cells of mice; there were some inteneration regions, incrassation of soft meninges, permeance of lymphocytes, chronic inflammation around a few blood vessels, deep-dyeing nerve cells of pallium, solidifying and shrinking of part of the nerve cell nucleus, and necrosis of a few nerve cells in most samples (9/10) of the CCNU 800 µg P.O. group, indicating that the traditional administration had severe damage effects on the nerve cells of mice. shows nerve tissue slices through the electronic telescope.
Discussions
The toxicological experiments indicate that the planted CCUN controlled release film will bring significantly fewer toxic effects on the haematogenous function and the neural system than the traditional administration (P.O.); that is the purpose and significance of our preparation of the controlled release film, under the condition of the assured curative effects.
At present, to get a successful planted controlled release film delivery system, there are still several issues to be improved. The most important problem is how to confirm the effective and low toxicity CCNU dose of the film. Because of the nondirectional diffusion of drug from the planted film in vivo, it is very difficult to estimate the accurate percentage of the drug arriving at the tumor regions. So it is the key goal to accurately control the drug from the planted film arriving at the tumor regions, and decrease its toxic effect to the normal tissues around the tumor (De Rosa et al., [Citation2002]; Duncan et al., [Citation1987]; Mallery and Pei, [Citation2000]; Shacter et al., [Citation2000]).
References
- De Rosa G., Quaglia F., La Rotonda M. I. Poly(lactide-co-glycolide) microspheres for the controlled release of oligonucleotide/polyethylenimine complexes. J. Pharm. Sci. 2002; 91(3)790–799
- Duncan R., Kopeckova-Rejmanova P., Strohalm J. Anticancer agents coupled to N-(2-hydroxypropyl)methacrylamide copolymers. I. Evaluation of daunomycin and puromycin conjugates in vitro. Br. J. Cancer 1987; 55(2)165–174
- Han Rui. Traditional Chinese medicine and search for new antineoplastic drugs. J. Ethnopharmacology 1998; 24: 1
- Mallery S. R., Pei P. Kang. Controlled-release of doxorubicin from poly(lactide-co-glycolide) microspheres significantly enhances cytotoxicity against cultured AIDS-related Kaposi's sarcoma cells. J. Anticancer Res. 2000; 20(5A)2817–2825
- Shacter E., Williams J. A., Hinson R. M. Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood 2000; 96(1)307–313
- Sun Yong, Zhou Haiying, Zhang Qiqing, Wu Xiaoyu. Improved controlled release study of lomustine. Artificial cells, Blood substitutes, and Immobilization Biotechnology 2000; 28(2)173–180