Abstract:
Conjugate of bovine hemoglobin (bHb) and human serum albumin (HSA) was prepared. The product was simply composed of 89.7% one-to-one Hb-HSA conjugate, 6.0% oligomer of Hb and HSA, 3.5% unconjugated HSA and 0.8% unconjugated Hb, with an average molecular weight of 157 kD. The physicochemical characteristics were determined. Effects of single replacement on blood pressure and long-term survival of rats with 30% and 60% acute blood loss were studied, in comparison with Ringer-lactate solution, stroma-free hemoglobin (SFHb), 5% HSA in Ringer-lactate, whole blood and no resuscitation fluid. Results showed that Hb-HSA conjugate maintained the mean arterial pressure of rats to initial level with no pressor effect. Long-term effects of the replacement fluids on 30% bleeding rats showed that, for the group infused with Hb-HSA conjugate, histology of five major organs, heart, kidney, liver, spleen and lung, were essentially normal, similar to that of whole blood, while obviously renal side-effects appeared in other groups. The efficacy of the conjugate was further demonstrated by the resuscitation of lethal hemorrhagic shock rats (60% acute blood loss) with 100% survival rate (followed for 14 days), the same result as whole blood. The Hb-HSA conjugate can thus be another candidate for blood substitute in emergency.
INTRODUCTION
Research on creating a solution with the properties of human red blood cells as a blood substitute has been going on for more than half a century, aiming at solving the problem of emergent transfusion and virus infection [Citation[2-4], Citation[20]]. Stroma-free hemoglobin (SFHb) was first developed but later terminated because of its serious renal toxicity caused by the dissociation of tetrameric structure of hemoglobin (Hb) into αβ subunits. Perfluorocarbon emulsions can increase the amount of O2 carried in the blood, though at the cost of unwanted side-effects such as thrombocytopenia and flu-like symptoms [Citation[11]]. Whilst liposome encapsulating hemoglobin (Hb vesicles, HbV) has the advantage of easy regulation, at the same time, of important physicochemical properties, such as O2 affinity, viscosity, colloid osmotic pressure (COP) and O2 release rate [Citation[18]], the red blood cell is far more sophisticated, containing a cytoskeleton and enzyme and transport systems that ensure hemoglobin flexibility for its 120-day lifespan [Citation[3], Citation[21]]. Modification of Hb focuses on prolonging the vascular retention of SFHb and reducing its renal toxicity. An example is bis-(3,5-dibromosalicy1) fumarate (DBBF) intramolecularly cross-linked hemoglobin (DBBF Hb) developed by Baxter [7]. i stabilizes the tetrameric structure of Hb, but the most vexing problem is vasoconstration with short half-life. Polymerizing the intramolecularly cross-linked Hb [6] or modifying Hb with macromolecules, such as polyethylene glycol (PEG) [Citation[1]] or polysaccharide [Citation[19]] was carried out in order to increase the molocular size and so extend the circulatory duration of Hb, not without, however, the problem of the wide distribution of molecular weight. The above research is concentrated on simulating the function of oxygen carrying to produce red blood cell substitutes, but not real blood substitutes.
Serum albumin is the largest fraction of plasma proteins that performs functions such as balancing osmotic pressure, carrying nutrient substances, etc. Conjugate of serum albumin with hemoglobin might possess the properties of both proteins, and could be an ideal blood substitute. The preparation of bovine hemoglobin (bHb) with bovine serum albumin (BSA) was reported by our group [Citation[12]]. In this paper, we prepared a conjugate of bHb and human serum albumin (HSA) and performed animal test on this product. HSA has a better human compatibility than BSA. The conjugate also has an enlarged molecular weight that could extend the circulatory duration.
We used an improved process for preparing a product containing about 90% of one-to-one Hb-HSA conjugate with an average molecular weight of 157 kD. Physicochemical characteristics were determined and effects of single replacement on blood pressure and long-term effects of rats with 30% and 60% acute blood loss were investigated. The safety and efficacy of the conjugate was demonstrated on the model of lethal hemorrhagic shock rats. Other resuscitations using Ringer-lactate solution, stroma-free hemoglobin (SFHb), 5% HSA in Ringer-lactate, whole blood and no resuscitation fluid were compared as controls.
MATERIALS AND METHODS
Preparation of Purified Hemoglobin
Bovine blood was collected from cattle slaughterhouse containing 0.3% citrate sodium solution as anticoagulant. Red blood cells (RBC) were centrifuged, washed, and hemolyzed. The hemolyzate was processed through membrane separation followed by anion exchange chromatography (IEC) with media of Q Sepharose Big Beads (Amersham Bioscience, Sweden). Detail of the chromatographic process was described in our previous paper [Citation[13]].
Preparation of Hb-HSA Conjugate
Intramolecularly cross-linked Hb was first prepared by the reaction between glutaraldehyde (GA) and 20 mg/ml Hb at 4°C for 1 h in 20 mmol/L sodium acetate buffer, pH 5.7 (GA:Hb = 30:1, molar ratio). Then HSA was added at a molar ratio of 1:2 with Hb and reacted at 4°C for 9 h. The unreacted GA was inactivated by excess amount of lysine (10 equal mole to GA). The reacted solution was then performed through IEC column packed with media of DEAE-Sepharose Fast Flow. Unconjugated Hb passed through with the rinsing solution, 50 mmol/L phosphate buffer solution (PBS) pH 7.0, at a flow rate of 150 cm/h. The conjugate was eluted by 50 mmol/L PBS containing 0.1 mol/L NaC1, pH 7.0, at the same speed. The chromatography was monitored by on-line measurement of conductivity and UV absorption at 280 nm and 405 nm. Conjugates were collected according to the high absorption both at 280 nm and at 405 nm. The column was regenerated with 50 mmol/L PBS containing 1 mol/L NaC1 at a flow rate of 150 cm/h.
The collected conjugates were finally changed to the physiological buffer with a hemoglobin concentration of 50 g/dl by ultrafiltration at 4°C. The composition of the physiological buffer was in g/10L: NaC1 = 58.5; NaHCO3 = 36.1; NaH2PO4 ⋅ H2O = 4.4; KC1 = 3.7; CaCl2 ⋅ 2H2O = 2.2; MgCl2 ⋅ 5H2O = 0.8. The osmotic pressure of the buffer was 300 mOsm.
Analytical Methods
Osmotic pressure was measured in the process of RBC swelling and lysating by Fiske ONE-TENTM Osmometer (Fiske Associates, USA). The concentration of hemoglobin was determined by the conversion of hemoglobin into cyanmethemoglobin and detecting the absorption at 540 nm [Citation[8]]. The content of methemoglobin was determined by the adsorption of 630 nm according to the method of Evelyn [Citation[10]]. The activity of hemoglobin, in terms of P50 and Hill coefficient, was measured by HemoxTM Analyzer (TCS Scientific Corp., PA).
The isoionic point of modified Hb was determined by native isoelectrofocusing (IEF) using Phastsystem (Amersham Biotech, Sweden), Phast Gel IEF3-9, the marker varies from 3 to 10 (Amersham Biotech). After electrophoresis, the gel was stained with Coomassie blue R250 and analyzed by Bio-Rad Gel Documentation Systems (Gel Doc 2000TM).
Size-exclusion chromatography (SEC) analysis was conducted with an instrument equipped with three wavelength spectrophotometric detector (AKTA Purifier 10 FPLC System, Amersham Bioscience, Sweden) on SuperdexTM 200 column (Amersham Bioscience) at 280 nm and 405 nm. A mobile phase of 50 mM phosphate buffer, pH 6.4, containing 0.15 M NaC1 at a flow rate of 0.5 mL/min was used. SEC using a mobile phase of 10 mmol/L Tris HCI containing 1 mol/L MgC12, pH 7.0, was applied with the same FPLC system to detect whether the modified hemoglobin could dissociate into αβ subunits in physiological environment [Citation[14]].
Absolute weight-average molecular weights (Mw) of Hb-HSA conjugate was determined by the SEC coupled with a multi-angle laser light scattering (MALLS) detector. An effluent of SuperdexTM 200 column was monitored sequentially with a DAWN EOS light-scattering detector (Wyatt Technology Co., USA). The design of the DAWN EOS flow cell incorporates a fixed photodiode detector array, capable of measuring scattered light intensity simultaneously at 18 angles, and measuring the mean square radius of particles and the distribution of mass within the molecule at the same time.
Animal Experiments
According to Requirements for Abnormal Toxicity Test of Biologics in Chinese Requirements for Biologics (2000), five rats weighing 18–20 g and two guinea pigs weighing 270–280 g were given 0.5 ml and 5 ml Hb-HSA, respectively, by abdomen injection and fed for seven days to test the safety of the modified Hb. All animals being alive and active in the week will prove no abnormal toxicity of the product.
Anesthetized Sprague-Dawley rats weighing 240–260 g were used to evaluate the effect of Hb-HSA conjugate on hypotensive hemorrhage. Rats were anesthetized with thiobutabarbital (0.045 g/kg) and placed on a heating surgical table to maintain body temperature at 37°C. Polyethylene cannulas were filled with 300 U/ml heparin and placed in carotid and left femoral arteries to measure mean arterial pressure (MAP, Monitor oscilloscope 2G66 and Polygraph 302, NEC San-ei Instruments Ltd., Tokyo, Japan) and withdraw blood, respectively. Hb-HSA or control solutions were infused from the right femoral vein.
The 30% and 60% of total blood volume bleeding were involved in blood replacement. Blood was slowly withdrawn at the speed of 0.5 mL/min, and immediately thereafter the same volume of Hb-HSA conjugate or controls was infused at the same speed. We used Ringer-lactate solution equivalent to three times the volume of shed blood, stroma-free hemoglobin in Ringer-lactate solution, 5% HSA in Ringer-lactate solution, whole blood and no resuscitation fluid as controls.
For each volume of bleeding, there were 6 groups of rats with 6 rats randomly assigned to each group. MAP was continuously recorded throughout the experiment. After the completion of infusion, rats were returned to separate cages and monitored for 14 days. Those rats that lived for 14 days were considered as having “survived.” The time of death was recorded for those that died before this time.
Five days and 24 days after the infusion, some animals were sacrified in sequence. Kidney, liver, spleen, heart and lung were fixed in formalin for light microscopy.
Statistical Methods
Results were compared between the groups at each stage of the study by a one-way analysis of variance. If p-level lower than 0.05, significant difference is considered. Data were expressed as mean±standard deviation.
RESULTS AND DISCUSSION
Characteristics of Hb-HSA Conjugate
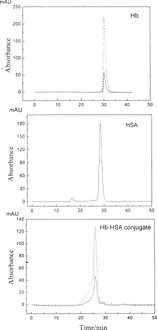
Figure shows SEC pattern of Hb-HSA conjugate. There was only one main peak accompanying with a few small peaks. Native hemoglobin has significant absorption at 405 nm for its heme, and the ratio of between A405 and A280 can reach to 4.5:1. HSA has no absorbance at 405 nm. For the main peak in Figure, the ratio of A405 and A280 decreased to about 2.8:1. Therefore, we can infer that the product was mainly composed of one-to-one Hb-HSA conjugate, not polyHb or polyHSA. To further determine the composition of the product, an advanced tool of MALLS coupled with SEC was applied. The composition of Hb-HSA conjugate was described in . The content of each component was calculated by comparison of peak areas. The final product of Hb-HSA conjugate was simply composed of 89.7% one-Hb-HSA conjugate with Mw148 kD, 6.0% oligomer of Hb and HSA, 3.5% unconjugated HSA and 0.8% unconjugated Hb. The average molecular weight of the Hb-HSA conjugate was 157 kD. Details of the preparation would be described elsewhere.
Table 1 Composition of Hb-HSA conjugate
Research on DBBF showed that cross-linking of α-α or β-β was necessary to stabilize the tetramer structure of Hb, because αβ subunits bonded tightly in native Hb and difficult to dissociate [Citation[7]]. Size-exclusion chromatography using a mobile phase containing 1 mol/L MgC12, pH 7.0, was applied to detect whether the modified hemoglobin would dissociation into αβ subunits in physiological environment [Citation[14]]. Results showed that the Hb-HSA conjugate did not dissociate into αβ subunits and the cross-linking stabilized the tetramer structure of Hb.
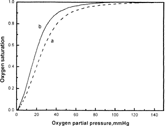
Figure describes the bioactivity variety of modified Hb. After modification, P50 and Hill coefficient of Hb-HSA conjugate decreased from 24.5 mmHg and 2.46 (purified Hb) to 18.2 mmHg and 2.10, respectively. Some reaction might change the molecule conformation of Hb, or affect the conformation changing from tense (T) to relax (R) state to liberate oxygen and so improve the oxygen affinity.
Although the same P50 as normal Hb was ideal for blood substitutes, several researchers claimed that high oxygen affinity (low P50) was more beneficial than low affinity (high P50). High oxygen affinity serves to preserve total O2 as blood approaches capillaries, but permits release in low tissue PO2 environments [Citation[21]], while low oxygen affinity makes the oxygen liberated before i comes into the tissues in need. i was reported that Hb-vesicles (HbV) with P50 = 16 mmHg showed better microcirculation than HbV with P50 = 30 mmHg after exchange transfusion [Citation[19]]. The appropriate oxygen affinities for O2 carriers have not been completed yet [Citation[18]], which need long-term clinical research.
Physiocochemical characteristics of RBC, Hb-HSA conjugate and other Hb-based O2 carriers in literature [Citation[18]] are shown in . Hb-HSA conjugate had similar properties as blood on oxygen carrying ability, viscosity, colloid osmotic pressure, etc. The conjugate had a moderate Mw and a simple composition, which could neither cause RBC aggregation or deteriorates microcirculation for high molecular weight like polyHb [Citation[15]], nor meet the problem of traversing vascular endothelium and glomerular capsule for low molecular weight like DBBF-Hb [Citation[16]], in addition with being charged opposite to that of endothelial cells of blood vessel and glomerular capsule for lower isoelectric point (pI) of 4.6–5.2.
Table 2 Physicochemical properties of RBC, Hb-HSA conjugate and other Hb-based O2 carriers
Toxicity of Hb-HSA Conjugate
Tests on rats and guinea pigs showed that the animals were all alive after seven days and grew normally with increasing weights, which indicated that the products had no abnormal toxicity.
Experiments on Rats
There is extensive literature on volume replacement in hemorrhagic shock. Many of these studies are based on bleeding approximate 30%–40% of blood volume. These are excellent models to study volume replacement, which is much more important than red blood cells' replacement in this situation. However, this model may not be sensitive enough to detect significant differences in efficacy between plasma expanders and blood substitute. Chang and Varma [Citation[5]] applied a lethal hemorrhagic shock rat model with 67% removal of total blood volume to show the efficacy of blood substitutes acting as both plasma expander and red blood cell replacement. In order to investigate the effect of the Hb-HSA conjugate on rats, we adopted both models, bleeding 30% and 60% of total blood volume, and compared the effects of Hb-HSA conjugate with other resuscitation fluids on acute response of blood pressure and survival. Ringer-lactate solution, SFHb, 5% HSA in Ringer-lactate, whole blood and no resuscitation fluid were used as controls.
Acute Response of Rats to Blood Replacement
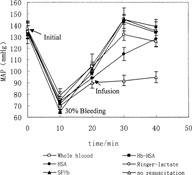
The short term MAP response of the animals after 30% removal of total blood volume is shown in Figure. After the completion of the 30% bleeding, MAP fell to 70% of baseline in the group with no resuscitation. For the two groups that received 3 volumes Ringer-lactate or 5% HSA in Ringer-lactate, MAP did not increase to the initial level. The blood pressure increased to higher than the initial level after infusing whole blood, SFHb, and Hb-HSA conjugate, and then decreased to a stable level. Hb-HSA conjugate maintained the MAP at initial levels, which showed no significant difference with whole blood according to statistical analysis. But for SFHb, the MAP increased from 132.2±4.0 mmHg to 139.3±4.1 mmHg. The above results showed that red blood cell replacements were better than volume replacements alone in maintaining MAP of 30% bleeding rats. The MAP increased about 5% after infusing SFHb. One speculated reason is that SFHb of this size is able to traverse the intercellular junctions of the endothelial lining of the blood vessels and bind nitric oxide needed for maintaining vasodilation, and cause vasoconstriction. The other reason is that the dissociation of hemoglobin into αβ subunits destroyed endothelial cell of blood vessel and glomeruli and increased the vasopermeability, and enhanced the adhesion of leucocyte [Citation[15]].
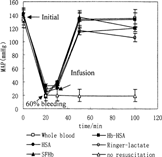
Figure shows the effects of 60% bleeding and replacement on the MAP of lethal hemorrhagic rats. In the group with no resuscitation, the blood pressure decreased to much lower than the initial value and stayed at this low level. Ninety minutes after the completion of 60% bleeding, all the animals in this group died. For the group that received 3 volumes Ringer-lactate, the MAP increased dramatically in the course of injection, but then decreased and did not return to the initial level. Similarly, 5% HSA in Ringer-lactate maintained the MAP at a lower level than the initial. Hb-HSA conjugate maintained the MAP to initial level in the hour monitored, having no significant difference with whole blood at the stage of before bleeding, after bleeding and after infusion. SFHb sustained the blood pressure only in the first 10 min and then decreased, which was different from the resuscitation of 30% bleeding rats with the same replacement fluid. For this SFHb group, a large amount of Hb appeared in urine 30–60 min after the injection. To the lethal hemorrhagic rats, renal effect for Hb dissociation is far more significant than pressor effect of SFHb for carrying NO out of vessels. Therefore, the compensation system is destroyed and difficult to maintain the MAP lever.
Long-Term Effects
Survival Rates
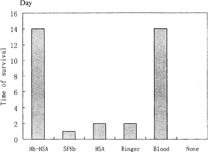
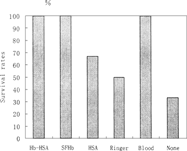
Effects of transfusion of the replacement fluids on the long-term survival of the animals were studied. The results of long-term survival (followed for 14 days) of 30% bleeding and 60% bleeding are shown in Figure and , respectively. For 30% bleeding, the survival rates of rats receiving 5% HSA and 3 Volumes Ringer-lactate were 66.7% and 50% respectively, higher than the group with no resuscitation. Volume replacement is important for self-compensation, but the efficiency is limited. Having the ability of carrying oxygen, whole blood, Hb-HSA conjugate and SFHb could help compensation system to keep balance of osmotic pressure and provide O2 for tissues with more efficiency in the resuscitation, resulting in all animals survived after infusion. Although SFHb showed the problems of short duration and renal effect, long-term survival demonstrated its efficacy with relative low dose of infusion, possessing advantage over volume replacements.
Figure 5 Survival rates of one single replacement transfusion (followed for 14 days) in 30% bleeding rat model.
After 60% acute blood loss, rats were in hemorrhagic shock with low MAP and microcirculation malfunction. All rats in the group with no resuscitation died in less than 2 h. The three groups that received SFHb, Ringer-lactate and HSA survived that day, but all died the next day. The group received Hb-HSA conjugate all survived in the 14 days monitored, the same as whole blood. Figure shows the effect of one single replacement transfusion on the long term survivals (followed for 14 days) in the lethal hemorrhagic shock model with 60% bleeding, which indicated that the Hb-HSA conjugate was effective in hemorrhagic shock, similar to whole blood.
Analysis on Histology
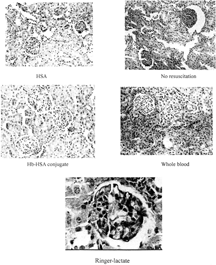
Tissue slices indicate the effects of replacement fluids on histology. In lethal hemorrhagic shock model with 60% bleeding, most rats died in two days, and the shock might affect the result. Therefore, we chose 30% bleeding of rats to evaluate the short terms effect (5 days) and long term effects (24 days) on five major tissues: heart, kidney, liver, spleen and lung.
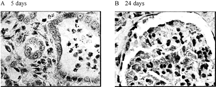
Under hypovolemic shock, the kidney is especially vulnerable to injury since its blood flow and function are compromised. Therefore, i was proposed that this may be a more sensitive test. As a result, the renal effect was the most significant, and histology of other organs was essentially normal except that some atrophied sinuses hepaticus appeared in those rats receiving SFHb for 5 days. Effects of SFHb transfusion on renal function for 30% bleeding rats are shown in Figure. Long term effects (24 days) of other replacement fluids on renal function are shown in Figure. Renal toxicity of SFHb has been reported in the literature [Citation[9], Citation[22]]. In our study, we investigated its short-term and long-term effects. Five days after the SFHb infusion, some phenomena such as glomerulus oedema, fibrocyte hyperplasia in renal medulla, and endothelial cell necrosis and neutrophile granulocyte hyperplasia in nephric tubules () appeared. Changes turned more severe after 24 days. Symptoms of glomerular nephritis were found, as mild interstitial infiltration, red blood cells and leucocyte appeared in glomerulus sacculus ().
Figure 7 Effects of SFHb transfusion on renal function for 30% bleeding rats. (A) Endothelial cell necrosis and neutrophile granulocyte hyperplasia in nephric tubules. (B) Red blood cells and leucocyte appeared in glomerulus sacculus.
Three groups, Ringer-lactate, 5% HSA and no resuscitation, had similar effects on kidneys (Figure). Glomerulus oedema appeared in those rats that had received infusion for five days. Twenty-four days later the phenomenon was not significant while glomerulus atrophy and decrease were observed. Especially for 5% HSA, nearly 20% was atrophied glomerulus, and the connected tubular turned edema, which led to the glomerulus nearby expanding for compensation. The rats with no resuscitation were affected the most seriously (). These effects might be attributed to lack of blood in microcirculation, shortage of oxygen in tissue or unbalance of colloid osmotic pressure in circulation system. In the case of Hb-HSA conjugate and whole blood, glomerulus oedema appeared in both groups after 5 days, which might be caused by temporary shock. Histology of kidneys was essentially normal after 24 days in both groups.
CONCLUSION
The Hb-HSA conjugate prepared in the present experiments had good physicochemical characteristics similar to blood. Effects of single replacement on blood pressure and long-term survival of rats with 30% and 60% acute blood loss demonstrated the advantages of Hb-HSA conjugate over SFHb and volume replacements. The conjugate maintained the MAP at its initial level with no pressor effect. Long-term effects of the replacement fluids on 30% bleeding rats showed that, to the group infused with Hb-HSA conjugate, histology of five major organs was essentially normal, similar to whole blood, but obvious renal side-effects appeared in other groups with Ringer-lactate solution, SFHb, 5% HSA in Ringer-lactate and no resuscitation fluid. The efficacy of the conjugate was further demonstrated by the resuscitation of lethal hemorrhagic shock rats (60% acute blood loss) with 100% survival rate (followed for 14 days), the same result as whole blood. The Hb-HSA conjugate can thus be another candidate as a blood substitute in emergency.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support received from the National Natural Science Foundation of China (No. 20136020) and from Key Project of Innovation Program by Chinese Academy of Sciences.
REFERENCES
- Baldwin, A.L., Wilson, L.M., Valeski, J.E. (1998). Ultrastructural effects of intravascularly injected polyethylene glycol-hemoglobin in intestinal mucosa. Am. J. Physiol. 275: H615–H625. [PUBMED], [INFOTRIEVE]
- Chang, T.M.S. (1964). Semi-permeable microcapsules. Science 146: 524–525. [PUBMED], [INFOTRIEVE]
- Chang, T.M.S. (1997). Blood Substitues: Principle, Methods, Products and Clinical Trials.Karger Publisher 1–141, Basel. (www.medicine.mcgill.ca/artcell/1972bookCovercr.pdf).
- Chang, T.M.S. (2000). Red blood cell substitues. Best Pract. Res. Clin. Haematol 13: 651–668. [CSA], [CROSSREF]
- Chang, T.M.S., Varma, R. (1992). Effect of a single replacement of one of ringer lactate, hypertonic saline/Dextran, 7g% albumin, stroma-free hemoglobin, o-raffinose polyhemoglobin or whole blood on the long term survival of unanesthetized rates with lethal hemorrhagic shock after 67% acute blood loss. Biomat. Art. Cells & Immob. Biotech 20(2–4): 503–510. [CSA]
- Cheng, D.C. (2001). Safety and efficacy of o-raffinose cross-linked human hemoglobin (Hemolink) in cardiac surgery. Can J Anaesth 48(4 suppl): S41–48. [PUBMED], [INFOTRIEVE], [CSA]
- D'Agnillo, F., Alayash, A.I. (2000). Site-specific modifications and toxicity of blood substitutes, the case of diaspirin cross-linked hemoglobin. Advanced Drug Delivery Reviews 40: 199–212. [CSA], [CROSSREF]
- Drabkin, D. (1949). The standardization of hemoglobin measurement. Am. J. Med. Sci 217: 710–711.
- Dubrow, A., Flamenbaum, W. (1988). Acute renal failure associated with myoglobinuria and hemoglobinuria, inAcute Renal Failure, B.M. Brenner, J.M. Lazarus, Eds.,New York: Churchill Livingstone, 279–293
- Evelyn, K.A., Malloy, H.T. (1938). Microdetermination of oxyhemoglobin, methemoglobin, and sulfhemoglobin in a single sample of blood. J. Biol. Chem 126: 655–662.
- Flaim, S. (1997). Perflubron-based emulsion: efficacy as temporary oxygen carrier, inAdvances in Blood Substitutes, Industrial Opportunities and Medical Challenges, R.M. Winslow, K. Vandegriff, M. Intaglietta, Eds.,Boston: Brikhäuser.
- Hu, T., Su, Z.G. (2003). Solid phase adsorption method for preparation of bovine serum albumin-bovine hemoglobin conjugate. Journal of Biotechnology 100(3): 267–275. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lu, X.L., Zhao, D.X., Su, Z.G. (2004). Purification of hemoglobin by ion exchange chromatography in flow-through mode with PEG as an escort. Artif. Cells, Blood Substit. and Biotechnol 32(2): 209–227. [CROSSREF]
- Manning, L.R., Morgan, S., Beavis, R.C., Chait, B.T., Manning, J.M., Hess, J.R., Cross, M., Currell, D.L., Marini, M.A., Winslow, R.M. (1991). Preparation, properties, and plasma retention of human hemoglobin derivatives: comparison of uncrosslinked carboxymethylated hemoglobin with crosslinked tetrameric hemoglobin. Proc. Natl. Acad. Sci. USA 88(8): 3329–3333. [PUBMED], [INFOTRIEVE], [CSA]
- Palaparthy, R., Wang, H., Gulati, A. (2000). Current aspects in pharmacology of modified hemoglobins. Advanced Drug Delivery Reviews 40: 185–198. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Przybelski, R.J., Daily, E.K., Kisicki, J.C., Mattia-Goldberg, C., Bounds, M.J., Colburn, W.A. (1996). Phase I study of the safety and pharmacologic effects of diaspirin cross-linked hemoglobin solution. Crit. Care Med 24: 1993–2000. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sakai, H., Tsai, A.G., Rohlfs, R.J., Hara, H., Tsuchida, E., Intaglietta, M. (1999). Microvascular responses to hemodilution with Hb vesicles as red blood cell substitutes: influence of O2 affinity. Am J. Physiol 276(Heart Circ. Physiol.45): 553–562.
- Sakai, H., Yuasa, M., Onuma, H., Takeoka, S., Tsuchida, E. (2000). Synthesis and physicochemical characterization of a series of hemoglobin-based oxygen carriers: objective comparison between cellular and acellular types. Bioconjugate Chem. 11: 56–64. [CSA], [CROSSREF]
- Schneeberger, E.E. (1988). Interaction of plasma proteins with negatively charged sites on the pulmonary capillary endothelium of the rat. Cell Tissue Res 251: 417–423. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Winslow, R.M. (2000). Blood substitutes. Advanced Drug Delivery Reviews 40: 131–142. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Winslow, R.W. (2003). Current status of blood substitute research: towards a new paradigm. Journal of Internal Medicine 253: 508–517. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Winslow, R.M. (1993). The toxicity of hemoglobin, in Hemoglobin-Based Red Blood Cell Substitutes,R.M. Winslow, Ed.,Baltimore: John Hopkins University Press, 136–163
- Winslow, R.M., Tsai, A.G., Vandergriff, K.D., Intaglietta, M. (2003). Targeted O2 delivery by low-P50 hemoglobin: a new basis for hemoglobin-based oxygen carriers. Artificial Blood 11(1): 45.