Abstract
Some “blood substitutes,” such as human diaspirin cross-linked hemoglobin DBBF-Hb, can damage the intestinal mucosa. This response may be due to release of free iron from Hb leading to production of reactive oxygen species (ROS). Phosphorothioate oligodeoxynucleotides can bind and sequester iron. Therefore experiments were performed to test whether PS-ODN, composed of ten consecutive cytidines “C-10,” reduces Hb-induced ROS generation and damage in the mucosa. Anesthetized Sprague-Dawley rats (4–6 per group) were injected arterially with 1 mg C-10, followed two minutes later by 50 mg DBBF-Hb. The positive controls received only DBBF-Hb and the negative controls either saline or PS-ODN followed by saline. Either ROS formation was monitored using a fluorescence technique, or the intestine was fixed for microscopy after 8 or 30 min. Sixty villi per rat were assigned an epithelial integrity index (EI), ranging from 1 (intact) to 3 (some cell–cell and cell–basement membrane separation). Pretreatment with PS-ODN significantly exacerbated DBBF-Hb-induced ROS formation, and PS-ODN groups showed significantly more epithelial damage near Peyer's patches, (EI of 1.93 ± 0.06 (SEM) at 8 minutes and 1.31 ± 0.04 at thirty minutes), than the negative controls, (1.11 ± 0.02 at both 8 and 30 minutes), or the positive controls (1.43 ± 0.05 at 8 minutes and 1.20 ± 0.03 at 30 minutes) ( p < 0.05). However, mast cell degranulation, eosinophil accumulation and goblet cell secretion were significantly reduced in the DBBF-Hb groups pre-treated with PS-ODN. Thus, PS-ODN, although an iron chelator, can significantly enhance epithelial damage caused by DBBF-Hb in the rat intestinal mucosa near Peyer's patches, possibly by formation of the ferryl component of the hemoglobin.
INTRODUCTION
Cell-free hemoglobin-based oxygen carriers, such as diaspirin cross-linked hemoglobin (DBBF-Hb), have been proposed as blood substitutes for transfusions due to their plasma expansion and oxygen transport capabilities. DBBF-Hb, produced by the US Army, is similar to DCLHb, the commercial analog that was produced by Baxter Health Care, Inc. A number of largely unresolved problems were found during pre-clinical trials and development of some of these hemoglobin-based substitutes. Baxter has recently terminated its clinical development due to increased fatalities in the test group [Citation[1]]. In addition, numerous animal studies have demonstrated that the administration of extracellular hemoglobin derivatives may lead to a variety of undesirable side effects [Citation[2-5]].
Previously, we showed that bolus injection of DBBF-Hb and polyethylene glycol (PEG)-conjugated Hb, another Hb-based blood substitute, cause transient ultrastructural alterations in the intestinal epithelium [Citation[6], Citation[7]]. These changes include detachment of intestinal epithelial cells from each other, and from the basement membrane, degranulation of mucosal mast cells and the activation of mucin-secreting goblet cells. The cellular mechanisms by which these deleterious effects occur are not known. However, one possibility is that the damage is mediated by reactive oxygen species (ROS) that are produced as a result of release of free iron from the modified hemoglobin. It is well known that modified hemoglobins spontaneously autooxidize to methemoglobin in vivo. For example, in guinea pigs it was shown that methemoglobin formation increased linearly up to a plateau of 30–40% at 12 hours following infusion of a hemoglobin conjugated to carboxylate dextran [Citation[8]]. Hydrogen peroxide then stimulates the release of free iron from methemoglobin [Citation[9]]. In addition, free hemin is generated during the normal catabolic degradation of administered hemoglobin, and the release of free iron from hemin is promoted by H2O2 [Citation[9]]. Evidence for a high rate of hemoglobin catabolism in vivo has been presented by Everse and Hsia [Citation[10]], who used data from Hess [Citation[11]] regarding the rate of disappearance of injected cross-linked hemoglobin from the circulation, taking into account the rate of extravasation.
If free iron is released in the bloodstream it catalyzes production of ROS by the Fenton reaction [Citation[12]]:The products of this reaction are ferric iron, the hydroxyl radical (˙OH) and the hydroxide ion. Fenton-type oxidations of organic substrates proceed via addition of ˙OH, or via hydrogen ion abstraction, as shown below:
In this way, tissue damage is initiated.
There are many examples of hemoglobin-induced tissue damage that have been shown to result, at least partially, from action of ROS produced by free iron. When the free radicals are inactivated with superoxide dismutase and/or catalase, or the free iron is chelated, the damage is reduced. For example, Paller [Citation[13]] showed that the iron chelator, desferroxamine, reduced renal dysfunction and accompanying ROS-mediated lipid peroxidation during heme-protein-induced acute renal failure. In an ovine model of exchange transfusion, metHb concentrations of a gluteraldehyde-polymerized bovine hemoglobin increased from 3% to 40% in only 24 hours [Citation[14]]. Desferrioxamine also prevents oxyHb-induced endothelial and smooth muscle cell cytoskeletal injury [Citation[15]]. However, there is little published information on the effect of iron chelation on the tissue damage induced by hemoglobin-based blood substitutes. Such information would clarify whether or not the Fenton reaction plays a major role in the oxidative tissue damage caused by hemoglobin-based blood substitutes and would aid in the development of superior products. If the Fenton reaction is responsible for the damage to the intestine produced by modified hemoglobins, then chelation of the free iron should reduce the damage.
For this reason, experiments were designed to determine whether intravenous injection of an iron chelator, prior to a bolus injection of DBBF-Hb, reduced the epithelial disruption, mast cell degranulation, eosinophil accumulation and Goblet cell secretion observed in the intestinal mucosa of rats [Citation[7]]. The chelator used in these experiments was a C-10 phosphorothioate oligodeoxynucleotide (PS-ODN) developed by AVI BioPharma, Corvallis, OR. This product has been shown to have a high affinity for iron and facilitates iron excretion [Citation[16]]. The reason that PS-ODN was used instead of the more widely used chelator, desferrioxamine, is that treatment with desferrioxamine is costly, requires parenteral administration and has side effects associated with its use.
MATERIALS AND METHODS
Hemoglobin Solution
The human hemoglobin cross-linked by bis(3,5 dibromosalicyl)fumarate (DBBF-Hb) was a kind gift from the Walter Reed Army Institute of Research, Washington, DC. A spectral analysis of hemoglobin oxidation products was performed using a Beckman DU640 spectrophotometer. The ratio of oxy (Fe2+) to met (Fe3+) forms of hemoglobin was calculated as previously described (Citation[17]) and found to be 0.21. The DBBF-Hb was filtered using a Sephadex G-50 column that was equilibrated with Hepes buffered saline, pH 7.4. The molarities of the components of the Hepes buffered saline were as follows: Hepes sodium salt (10 mM), Hepes acid (11 mM), NaCl (132 mM), KCl (4.7 mM), CaCl2 (2.0 mM), and MgSO4 (1.2 mM), giving a total molarity of 160.9 mM, and a total osmolarity of 323.8 mosmoles. To increase the concentration of oxy to met Hb, the DBBF-Hb was reduced by adding sodium dithionite (50 mg/100 ml). The sample was then immediately applied to a Sephadex G-50 column, and the eluant, recognized as reduced Hb by its cherry-red color, was collected. Spectral analysis of this solution gave a ratio of 6.8 oxy to met forms of Hb. All hemoglobin solutions were equilibrated with room air during the experiments. However, the concentration of hemoglobin used in these experiments was less than one–tenth of that found in blood, and in addition, oxygen has a low solubility in water. Therefore, the tissues were not exposed to higher concentrations of oxygen than they would experience in vivo.
Pre-Experimental Treatment of Rats
Male Sprague Dawley rats, weighing 350–400 g, were obtained from Harlan. Monthly serology, bacteriology and parasitology evaluations are performed on animals from each virus-free barrier at Harlan. The rats were transported to the animal facility at Tucson VAMC by truck. The animal facility is small with a low personnel activity, and monthly tests are performed on sentinel rats. On arrival the animals were housed two per cage in a room (3 m by 4 m) deliberately chosen so as to be remote from noisy air vents and cage washers, etc. The cages were 45 cm long and 24 cm wide and contained standard Harlan sanichip bedding. Ten to twenty rats were housed in the room at any given time and no other rats, apart from those participating in this study, were housed with them. A technician entered both rooms once a day to feed and tend to the rats. The temperature ranged between 72 and 74°F, and the humidity was kept between 55 and 60%. The rats were fed Harlan Tech Lab 485 rat chow, and placed on a light cycle with lights on between 6:00 a.m. and 6:00 p.m.
Anesthesia
Sprague Dawley rats were pre-anesthetized with 1 mg/kg body weight of the following mixture: ketamine hydrochloride (5 ml of 100 mg/ml), acepromazine maleate (2 ml of 10 mg/50 ml) and xylazine (8 ml of 20 mg/ml). This was followed by an intraperitoneal injection of sodium pentobarbital (30 mg/kg). In each rat a tracheostomy was performed for artificial ventilation.
Procedures
The following procedures were performed to test the hypothesis that iron released from DBBF-Hb catalyses production of ROS, which then causes tissue damage that is mediated, in part, by mast cell degranulation. First (procedure #1), experiments were carried out to determine the amount of iron released in the circulation in vivo by a bolus injection of DBBF-Hb. Next (procedure #2), this amount of iron was injected into the circulation of rats to compare its effects on cells in the intestinal mucosa with those previously observed following injection of DBBF-Hb. Effects of pretreatment with PS-ODN on cellular changes induced by DBBF-Hb were also determined. Next (procedure #3), the effect of pretreatment with the iron chelator, PS-ODN, on production of ROS in intestinal mucosa by DBBF-Hb, was examined. Finally (procedure #4), the reactions of PS-ODN with DBBF-Hb, in the presence and absence of H2O2, were evaluated using spectrophotometry to determine the relative amounts of different hemoglobin derivatives formed in each case.
Procedure 1. To Determine Amount of Iron Released In Vivo by Injection of DBBF-Hb
a. Surgical Procedures
After anesthesia, a mid-line abdominal incision was made to expose the aorta. The aorta was cannulated just downstream from the superior mesenteric artery in a retrograde direction. The free end of the catheter tubing was connected to a reservoir of HBS-2% BSA, pH 7.4, at 37°C. DBBF-Hb (50 mg in 5 ml HBS-2% BSA) (6 rats) or HBS-2% BSA alone (6 rats) was injected through a 0.2 micron filter via the aortic cannula. Blood samples (1 ml) were collected from the aortic cannula in heparinized vials 15 min. and 30 min. after the injection. The samples were immediately centrifuged, the hematocrits noted, and the supernatants aspirated and frozen. Meanwhile, the animal was prepared for a different study before sacrificing it using an intravenous injection of Beuthanasia.
b. Data Acquisition
In order to determine the concentration of free iron in the samples, a total iron-binding capacity kit (sigma-Aldrich Fine Chemicals, #565-C) was used. This technique measures total non-hemoglobin-bound iron, whether free or derived from heme. At acid pH and in the presence of a reducing agent, transferrin-bound iron dissociates and reacts with ferrozine, forming a magenta complex. The color intensity at 560 nm (37°C), read using a spectrophotometer, is proportional to the serum total iron concentration. A standard curve was constructed using a set of iron standards (Sigma-Aldrich Fine Chemicals). Then the experimental samples were thawed, concentrated to 250 µl using an Amicon centriprep concentrator, and the kit directions followed to determine the total non-hemoglobin iron concentration. The values obtained were corrected for the fact that the samples had been concentrated.
c. Data Analysis
The amount of free iron present per ml of plasma was determined for each sample, and the data pooled between animals of a given group for each time point, to obtain means and standard deviations. Paired Student t-tests were performed to determine whether there was a significant difference between the samples obtained from animals injected with DBBF-Hb and those injected with HBS-2% BSA, 15 min. and 30 min. after injection. The amount of iron present in the circulation 30 min. after a bolus injection of DBBF-Hb, as estimated from these experiments, was injected into rats, in the form of iron-dextran, in a further set of experiments described below (procedure #2) to determine whether its effects on epithelial disruption, mast cell degranulation and Goblet cell secretion were similar to those produced by DBBF-Hb.
Procedure 2. Microscopy of Intestinal Mucosa to Assess Mast Cell Degranulation, Goblet Cell Secretion, Eosinophil Accumulation and Epithelial Damage
a. Surgical Procedures
The following study was performed to determine the intestinal tissue damage 30 min. after intravenous injection of iron-dextran (6 rats), and to compare this with the damage caused by 8- and 30-min. treatments with 50 mg DBBF-Hb, with and without pretreatment with PS-ODN (4–6 rats each). Control animals received either HBS-2% BSA for 30 min. (6 rats), or PS-ODN for 2 min. followed by HBS-2% BSA for 30 min. (3 rats). After anesthesia, a mid-line abdominal incision was made to expose the aorta. The aorta was cannulated just downstream from the superior mesenteric artery in a retrograde direction. The free end of the catheter tubing was connected to a reservoir of HBS-2% BSA, pH 7.4, at 37°C. A loop of intestinal ileum, close to the cecum, was pulled outside the body cavity and arranged on a plexiglas pillar attached to the plastic stage on which the rat was situated. In PS-ODN experiments, 1 mg PS-ODN in 0.5 ml HBS was injected through a 0.2 micron filter via the aortic cannula, followed two minutes later by 50 mg (10 mg/ml) DBBF-Hb in HBS-2% BSA, or just HBS-2% BSA. In the remaining experiments, the PS-ODN injection was omitted. The iron dextran was dissolved in HBS-2% BSA and its concentration was adjusted such that the amount of iron contained within the bolus was equivalent to that released by a bolus injection of DBBF-Hb. After 8 or 30 min., the aorta was clamped upstream of the superior mesenteric artery and the intestinal circulation was perfused via the aortic cannula with Karnovsky's fixative in phosphate buffer, pH 7.4, at 4°C. When perfusion was complete, the inlet pressure was dropped to 40 mmHg and the portal vein was clamped. The animal was killed with an intravenous injection of Beuthanasia. Fixation continued for 60 minutes after which the intestinal loop was excised and cut into several segments, each containing a Peyer's patch; these segments were washed in buffered saline.
b. Tissue Preparation for Histology
Tissue squares were immersed in diaminobenzidine (DAB) overnight in the dark to stain specific granules in eosinphils, and thus make the cells easier to identify. The DAB was prepared as follows [18]: DAB (0.1 g) was added to 50 ml 0.1 M monobasic phosphate buffer and the pH was adjusted to 7.2 very gradually with concentrated NH4OH. The solution became a light tannish-pink color. Next, the tissue squares were rinsed in distilled water. Meanwhile, 25 ml DAB solution was added to 1.66 ml 3% H2O2 to give final concentration of 0.2%. The tissue was placed in this solution for 60 minutes and then rinsed three times in 0.15 M sodium cacodylate buffer. Finally, the tissue was dehydrated in increasing concentrations of ethanol and embedded in Spurrs resin. The pieces of tissue were oriented in the resin so that the blocks could be sectioned perpendicular to the villus plane. Thick sections (2 µm) were cut for light microscopy, mounted on slides, and stained with toluidine blue. Ultrathin sections were cut for electron microscopy (Phillips CM12). Before examining the sections under electron microscopy, the grids were stained with lead citrate and uranyl acetate.
c. Data Analysis
Thick sections were examined under light microscopy to count the numbers of degranulated mast cells secreting goblet cells, and eosinophils per villus cross section, in 30–60 villi per animal taken from four different regions of the tissue sample. Only villus sections that contained a central lacteal were included because these villi were centrally sectioned. Mast cells were considered degranulated if they exhibited empty vacuoles. Eosinophils were recognized by the DAB-stained granules (reddish tinge), together with the toluidine blue stained cytoplasm. For collection of data the slides were coded and the code was not revealed until the data were analyzed, as previously described, using n as the number of villi in each group. Electron microscopy (×3000) was used to assess the integrity of the epithelium (E.I.) in each villus cross section examined. Intact epithelium was assigned an E.I. of 1; epithelium with some cell-cell separation was assigned 2, and epithelial cells detaching from the basement membrane scored 3.
Procedure 3. In Vivo Estimation of Reactive Oxygen Species in Intestinal Mucosa
a. Surgical Procedures
Experiments were performed on 10 rats (5 with PS-ODN pretreatment and 5 without). After anesthesia, the femoral artery was cannulated in a retrograde direction to blood flow. The free end of the catheter tubing was connected to a syringe of HBS-2% BSA, pH 7.4, at 37°C. Next, a mid-line abdominal incision was made and a loop of intestinal ileum, close to the cecum and containing a Peyers' patch, was pulled outside the body cavity. The segment of intestine was arranged on a plexiglas pillar attached to the plastic stage on which the rat was cauterized longitudinally and the mucosal surface spread over a plexiglass. The mucosal segment was kept moist with a HBS- 0.5% BSA drip heated to 37°C, and autofluorescence from the tissue was recorded from six to eight villi surrounding the Peyer's patch. Next, the HBS-BSA was replaced by HBS-BSA containing 0.001 g/dl dihydrorhodamine 123 (DHR). This substance is not fluorescent until oxidized by a high energy radical. The rate of accumulation of the fluorescent product for a constant suffusate concentration of DHR provides a quantitative measure of oxidant formation in the tissue [Citation[19]]. An image of fluorescence from the same villi was recorded. This pre-heme-protein image served as a backgroud for later digital subtraction. Next, a 0.5 ml bolus of 1 mg PS-ODN in HBS-2% BSA (or just HBS-2% BSA for controls) was injected via the femoral cannula through a 0.2 µm filter, and a timer was started on the video monitor. Two minutes later, a 5 ml bolus of 10 mg/ml DBBF-Hb in HBS-2% BSA was injected by the same route. Every 2 minutes, for the next 30 minutes, several drops of DHR were applied to the preparation, and an epifluorescent image recorded. During acquisition of images, the following precautions were taken:
Each fluorescence observation was limited to a few seconds, after which a barrier filter was applied to block the fluorescence and prevent photobleaching.
The quantity of DHR that was dripped on the preparation between observations was carefully controlled so that the fluorescence intensity obtained was not affected by variations in DHR suffusate volume.
The offset and gain of the camera was kept constant for all experiments. Finally, the animal was sacrificed with an overdose of anesthetic.
b. Data Analysis
The mean intensity of the epithelial fluorescence of the villi contained in each image was measured using NIH-Image, and the background value (DHR before injection) subtracted. Data were pooled among the five animals in each group for each time point, and the means and standard deviations calculated. Similar measurements were made on the villus lamina propria. The data were then analyzed as described previously.
Procedure 4. Spectrophotometry of PS-ODN and DBBF-Hb In Vitro
Stock DBBF-Hb (200 mg/ml) was diluted × 80 in 1 ml HBS and a wavelength scan from 300–700 nm was performed using a Beckman spectrophotometer. Then, the same dilution of DBBF-Hb was mixed with PS-ODN to a weight ratio of 50:1, as was used in the in vivo experiments, and wavelength scans were performed 10 min. and 20 min. later. The whole procedure was repeated in the presence of 100 µM H2O2. It is known that production of H2O2 by activated neutrophils can reach concentrations as high as 100–600 µM [20]. Next, the Winterbourn equation [17] was used to calculate the percentages of oxy Hb, metHb, and ferryl Hb in each case. Ferryl heme, FeIV, is a highly reactive species that can peroxidize lipids, degrade carbohydrates and cross-link proteins and is produced by the reaction of oxyHb or metHb with H2O2 [Citation[21]].
RESULTS
Procedure 1. To Determine Amount of Iron Released In Vivo by Injection of DBBF-Hb
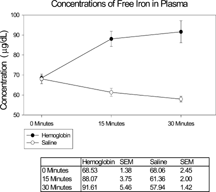
The concentrations of non-hemoglobin-bound iron detected in the blood samples taken after bolus injection of 50 mg DBBF-Hb in 5 ml HBS-2% BSA, or HBS-2% BSA alone, are shown in . After 15 min. there was significantly more iron in the samples as a result of the DBBF-Hb injection compared to control values (88.07 ± 3.75 (SEM) µg/dL vs. 61.36 ± 2.00 (SEM) µg/dL. After 30 min. the former value had risen slightly, and the latter value had fallen slightly, but not significantly. Therefore, in experiments to compare the effect of free iron on cells in the intestinal mucosa, with effects previously observed following injection of DBBF-Hb, a concentration of iron dextran was used that gave a plasma concentration of 90 µg/dL, assuming the observed hematocrit of 50% and a 30 ml blood volume in a 360–370 g rat.
Figure 1 Graph to demonstrate concentration of free iron in plasma derived from blood samples taken from rats at various times after bolus intravascular injection of 5 ml DBBF-Hb (50 mg) or isotonic saline. Error bars indicate SEM. After 15 min. there was significantly more iron in the samples as a result of the DBBF-Hb injection compared to control values (88.07 ± 3.75 (SEM) µg/dL vs. 61.36 ± 2.00 (SEM) µg/dL, p < 0.05.
Procedure 2. Microscopy of Intestinal Mucosa to Assess Mast Cell Degranulation, Goblet Cell Secretion, Eosinophil Accumulation and Epithelial Damage
a. Degranulated Mast Cells
Mucosal degranulated mast cells (dmc) were easy to identify by light microscopy because they stained intensely with toluidine blue and demonstrated empty vacuoles. A comparison of the degree of mast cell degranulation seen in the various groups is shown in . The numbers of villi from which degranulated mast cell counts were obtained ranged from 100 to 180. At villi near Peyers' patches, the mean numbers of degranulated mucosal mast cells observed per villus were significantly higher for animals treated with PS-ODN alone, DBBF-Hb for 8 and 30 min., and iron-dextran than for the HBS-2% BSA control group. Pre-treatment with PS-ODN significantly reduced mast cell degranulation in animals that received DBBF-Hb for 8 or 30 min. in villi both adjacent to, and removed from, Peyers' patches. Treatment with iron-dextran for 30 min. produced significantly less mast cell degranulation than did DBBF-Hb for the same time duration. These results indicate that mast cell degranulation in this system can at least partially be accounted for by the Fenton reaction.
Figure 2 Average number of intestinal mucosal degranulated mast cells per villus for different treatment. The treatments consisted of a 0.5 ml bolus injection of the first substance, followed 2 minutes later by a 5 ml bolus injection of the second substance. All experiments were terminated 30 minutes after the second injection. In all cases, the concentrations of DBBF-Hb, iron dextran and PS-ODN were 10 mg/ml, 0.9 µg/ml and 1 mg in 0.5 ml, respectively; *Significantly larger than saline control group, p < 0.05; #Significantly smaller than saline/DBBF-Hb group at same time point, p < 0.05.

b. Secreting Goblet Cells
A comparison of the extent of goblet cell secretion seen in the various groups is shown in . At villi near Peyers' patches, the mean numbers of secreting goblet cells observed per villus were significantly higher for animals treated with DBBF-Hb for 8 min. (without PS-ODN pre-treatment), with DBBF-Hb for 30 min. (with and without pre-treatment with PS-ODN), or with iron-dextran than for the HBS-2% BSA control group. Similar results were obtained remote from Peyer's patches except for the DBBF-Hb treatment for 8 min. without PS-ODN. Near Peyer's patches, pre-treatment with PS-ODN significantly reduced goblet cell secretion in animals that received DBBF-Hb for 8 min., but significantly increased goblet cell secretion after DBBF-Hb treatment for 30 min. Treatment with iron-dextran for 30 min. produced either more goblet cell secretion (near Peyer's patches) or the same amount (away from Peyer's patches) as DBBF-Hb for the same time duration. These results suggest that goblet cell secretion in villi near Peyer's patches may be stimulated by the Fenton reaction, but later on, in the presence of PS-ODN, another mechanism comes into play.
Figure 3 Average number of intestinal mucosal secreting goblet cells per villus for different treatments as described for Figure 2. In all cases, the concentrations of DBBF-Hb, iron dextran and PS-ODN were 10 mg/ml, 0.9 µg/ml and 1 mg in 0.5 ml; *Significantly larger than saline control group, p < 0.05; #Significantly different from saline/DBBF-Hb group at same time point, p < 0.05.

c. Eosinophil Accumulation
The average numbers of eosinophils per villus cross-section in rats perfused with (1) HBS-2% BSA, (2) DBBF-Hb for 8 min. and (3) DBBF-Hb for 8 min. after pretreatment with PS-ODN were 4.0 ± 2.5 (SD) ( n = 154), 9.6 ± 4.2 ( n = 84) and 5.7 ± 8.8 ( n = 37), respectively, where n is equal to the number of villi. The number of villi was lower for the third group because only some of the tissues were stained with diaminobenzoate, which is needed to demonstrate eosinophils. All three values were significantly different from each other. Thus, bolus injection of DBBF-Hb increases the number of eosinophils found within the lamina propria of intestinal mucosal villi, compared to controls, and pretreatment with PS-ODN significantly reduces this effect. The reduction in eosinophil recruitment caused by PS-ODN is consistent with the decrease in mast cell degranulation observed under the same conditions because mast cells, on degranulation, produce substances that attract eosinophils [Citation[22]].
d. Epithelial Damage
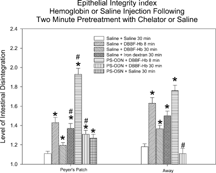
Electron micrographs of transverse sections through intestinal mucosa from rats perfused with HBS-2% BSA showed mostly intact epithelium (E), as seen in . An intact mast cell (MC) is also visible. On the other hand, a bolus injection of iron-dextran (producing an equivalent plasma concentration of iron as that released by a bolus injection of DBBF-Hb after 30 min. in the circulation) caused extensive epithelial disruption (, near Peyers' patch). The epithelial cells were sometimes separated from each other at the basal aspects of their intercellular junctions, and were linked only by long, cytoplasmic protuberances. In addition, the subepithelial interstitum was edematous, as evidenced by the large areas of electron-lucent space that were not seen in control preparations. Similar pathologies were seen in animals injected with DBBF-Hb for 8 min. (not shown). Pre-treatment with PS-ODN did not improve matters 8 min. after injection of DBBF-Hb (, away from Peyers' patch). In fact, near Peyers' patches, the areas of epithelial disruption were even more extensive than without the pretreatment. Thirty min. after bolus injection of DBBF-Hb, there was some repair of the epithelium (, away from Peyers' patch). The epithelial cells drew closer together and the cytoplasmic protuberances retracted. However, in villi near Peyers' patches, pretreatment with PS-ODN inhibited this repair (, Peyers' patch). In villi removed from Peyers' patches, the repair was significantly improved by PS-ODN at this timepoint compared to that observed with DBBF-Hb alone. The average Epithelial Integrity indices for each group are depicted in . Near Peyers' patches, iron-dextran for 30 min., and PS-ODN alone, significantly increased E.I. compared to HBS-2% BSA controls. In addition, pretreatment with PS-ODN significantly enhanced epithelial damage seen both at 8 min. and at 30 min. after DBBF-Hb injection. However, in villi away from Peyer's patches pre-treatment with PS-ODN, significantly enhanced epithelial integrity (lower E.I.) observed 30 min. after DBBF-Hb injection compared to that observed without pre-treatment. These results suggest that the Fenton reaction is partly responsible for epithelial disruption in this system, but that near Peyer's patches, in the presence of PS-ODN, another mechanism comes into play.
Figure 4 Electron microscopy of intestinal mucosa to assess epithelial damage. Typical electron micrograph of transverse section through intestinal villus (away from Peyers' patch) from rat injected with a 5 ml bolus of HBS-2% BSA (Hepes buffered saline containing 2% bovine serum albumin). The epithelium (E) is mostly intact and an intact mast cell (MC) is visible. Capillary cross-sections (C) can also be seen. Villi close to Peyers' patches were similar in appearance; Scale bar: 5 microns.
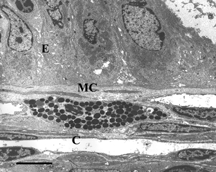
Figure 5 Electron microscopy of intestinal mucosa to assess epithelial damage. Typical electron micrograph of transverse section through intestinal villus (Peyer's patch area) 30 minutes after injection of rat with a 5 ml bolus of iron dextran (0.9 µg/ml). The epithelial cells (E) are separating from each other and from the basement membrane (arrows) and show extensive cytoplasmic protrusions. The interstitium, (I) is edematous. C: capillary containing two red blood cells; Scale bar: 5 microns.
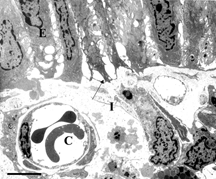
Figure 6 Electron microscopy of intestinal mucosa to assess epithelial damage. Typical electron micrograph of transverse section through intestinal villus (away from Peyer's patch) from rat that had been injected with 1 mg PS-ODN in 1 ml, followed 2 minutes later by 50 mg DBBF-Hb in 5 ml. The experiment was terminated eight minutes after the DBBF-Hb injection. Similar to the results of injection with iron dextran (Figure 5) and with DBBF-Hb alone (not shown), the epithelial cells (E), with extensive cytoplasmic protrusions, are separating from each other and from the basement membrane (arrows). Near Peyers' patches, the areas of epithelial disruption were even more extensive than with DBBF-Hb alone. C: capillary containing two red blood cells. Scale bar: 5 microns.
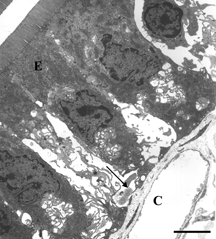
Figure 7 Electron microscopy of intestinal mucosa to assess epithelial damage. Typical electron micrograph of transverse section through intestinal villus (away from Peyer's patch) from rat that had been injected with 0.5 ml HBS-2% BSA, followed 2 minutes later by 50 mg DBBF-Hb. The experiment was terminated 30 minutes after the DBBF-Hb injection. The epithelial cells (E) are much closer together than after 8 minutes perfusion with DBBF-Hb, and the cytoplasmic protuberances are much less evident, indicating some repair of the epithelial lining. Villi near Peyer's patches were even more intact. Scale bar: 5 microns.
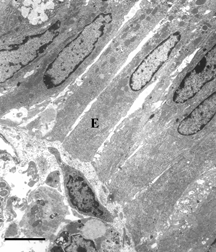
Figure 8 Electron microscopy of intestinal mucosa to assess epithelial damage. Typical electron micrograph of transverse section through intestinal villus (Peyers' patch area) from rat that had been injected with 1 mg PS-ODN in 0.5 ml, followed 2 minutes later by 50 mg DBBF-Hb in 5 ml. The experiment was terminated 30 minutes after the DBBF-Hb injection. Unlike the villi from animals injected with HBS-2% BSA for 2 minutes followed by DBBF-Hb for 30 minutes (Figure 7), the villous epithelial cells (E) are separated from each other and show extensive cytoplasmic protuberances. A degranulated mast cell (DMC) is visible. Thus pretreatment with PS-ODN appears to inhibit epithelial repair of villi adjacent to Peyer's patches. Scale bar: 5 microns.
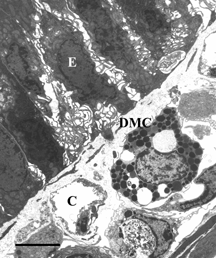
Figure 9 The average Epithelial Integrity indices for each group. The scale ranges from 1 to 3; a score of 1 means the cells are intact, 2 means the cells show some separation from each other, and 3 means there is some separation of cells from the basement membrane; *Significantly larger than corresponding saline control group, p < 0.05; #Significantly different from saline/DBBF-Hb group at same time point, p < 0.05.
Procedure 3. In Vivo Estimation of Reactive Oxygen Species in Intestinal Mucosa
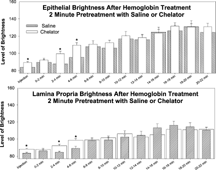
In preliminary experiments, in which no systemic injection was given to the animal, and the mucosal epithelium was exposed and suffused with DHR 123, the background fluorescence was low and did not increase over a time period of 30 min. When rats were injected with DBBF-Hb after a 2 min. pretreatment with HBS-2% BSA, the fluorescence in the epithelium, and in the lamina propria, rapidly increased within the 22 min. observation period (). A similar response was observed after pretreatment with PS-ODN, except that the level of brightness of the fluorescence was significantly greater for both the epithelium and the lamina propria at the time of DBBF-Hb injection and 2–6 min after injection.
Figure 10 In vivo relative estimation of reactive oxygen species in intestinal mucosa. Histogram to show fluorescence intensity of dihydrorhodamine 123 in the epithelium (upper panel) and lamina propria (lower panel) of intestinal villi after injection of DBBF-Hb (5 ml bolus at 10 mg/ml), following pre-treatment with saline (0.5 ml HBS-2% BSA for 2 minutes) or chelator (0.5 ml PS-ODN (1 mg) for 2 minutes). On the abscissa is plotted the time after injection of DBBF-Hb in minutes. Asterisks signify value is significantly greater for chelator pre-treatment than for saline pre-treatment.
Procedure 4. Spectrophotometry of PS-ODN and DBBF-Hb In Vitro
The percentages of oxyHb, metHb and ferrylHb in samples of DBBF-Hb, with and without addition of PS-ODN, in the absence and presence of H2O2, are presented in . Addition of PS-ODN to the DBBF-Hb increased oxyHb and decreased metHb and ferrylHb, all very slightly. Addition of H2O2 to the DBBF-Hb dramatically decreased oxyHb and increased both metHb and ferrylHb. The increased ferryl component of DBBF-Hb that forms after reaction with H2O2 has been reported previously [Citation[23]]. Addition of H2O2 to DBBF-Hb, but in the presence of PS-ODN, decreased oxyHb and increased ferrylHb to an even greater extent than without PS-ODN. For example, 20 minutes after addition of H2O2, the percentage of oxyHb was 23.8% without PS-ODN, and 16.1% with PS-ODN, while the corresponding values for ferrylHb were 49.2% and 59.0%, respectively. These results indicate that, at least in vitro, PS-ODN increases the oxidation state of DBBF-Hb in the presence of H2O2.
Table 1. Percentages of different oxidation states of DBBF-Hb under various conditions
DISCUSSION
The results from this study indicate that release of free iron by DBBF-Hb after bolus injection in rats, causes significant mucosal mast cell degranulation in villi adjacent to Peyers' patches, eosinophil accumulation in the intestinal villus lamina propria, goblet cell secretion, and epithelial disruption. It is probable that the increased accumulation of eosinophils in the intestinal mucosa was activated by the release of eosinophil activating factors from the degranulating mast cells [22]. Pre-treatment of rats with an iron chelator, the oligonucleotide PS-ODN, prior to bolus injection of DBBF-Hb, in an effort to chelate the free iron, resulted in a significant reduction in mast cell degranulation, and in goblet cell secretion and eosinophil accumulation in villi adjacent to Peyers' patches, observed 8 min. after injection, compared to rats receiving DBBF-Hb without PS-ODN. These results suggest that the DBBF-Hb-induced mast cell degranulation, goblet cell secretion and eosinophil accumulation are mainly triggered by the release of free iron, which subsequently catalyzes the Fenton reaction to produce the hydroxyl radical (OH). The fact that injection of iron dextran did not cause quite as much mast cell degranulation as was evident 30 min. after injection of DBBF-Hb suggests that another mechanism may also have been involved in the case of the blood substitute. However, this other component was minor because iron chelation reduced mast cell degranulation almost to control levels.
Although treatment with PS-ODN significantly reduced mast cell degranulation, goblet cell secretion and eosinophil recruitment, it markedly increased epithelial disruption near Peyers' patches. The mechanisms responsible for the increased epithelial disruption were suggested by the in vivo experiments to measure generation of ROS in the epithelium and lamina propria, and by the in vitro spectrophotometric measurements. Measurements of the fluorescence intensity produced by DHR123 showed that generation of ROS for up to 6 min. after bolus injection of DBBF-Hb was significantly increased by pretreatment with PS-ODN, both in the epithelium and in the lamina propria. It is known that DHR123 fluoresces in response to H2O2 [Citation[19]]. As explained in a previous publication [Citation[24]], excess hydrogen peroxide may accumulate in the intestinal mucosa even in response to intravenous injection of buffered saline, possibly due to dilution of the oxidant scavenger, catalase, in the plasma. In addition, H2O2 can be produced by activated neutrophils [Citation[25]]. Neutrophils are present in the lamina propria of intestinal villi and they may be the source responsible for the excess H2O2 that was produced in the presence of PS-ODN in the lamina propria. In addition, oxidation of Hb to metHb produces superoxide (O2−), which then reacts with superoxide dismutase to produce H2O2. A higher rate of auto-oxidation of DBBF-Hb in the presence of PS-ODN could therefore explain an increased production of H2O2. In the spectrophotometric measurements, it was found that metHb production, and hence oxidation of Hb, was increased by PS-ODN in the absence of H2O2. Therefore, perhaps, at the start of the bolus injection of DBBF-Hb when H2O2 in the epithelium is low, the PS-ODN increases the oxidation rate of the DBBF-Hb, forming O2− which is dismutased to H2O2. As the concentration of H2O2 increases, the H2O2 may start reacting with the metHb and oxyHb, transforming them to ferrylHb, and this may explain why the higher accumulation of ROS in the mucosa of rats pretreated with PS-ODN did not persist past 6 minutes after injection of DBBF-Hb. This idea is consistent with the spectrophotometric measurements in which it was found that in the presence of H2O2, the formation of ferrylHb was enhanced by PS-ODN. Since DBBF-Hb-induced epithelial damage near Peyers' patches was worsened in the presence of PS-ODN, this indicates that the epithelium may be particularly vulnerable to ferrylHb. However, the release of free iron also appears to cause epithelial damage by the Fenton reaction as demonstrated by bolus injection of iron dextran. Therefore, injection of DBBF-Hb probably damages the epithelium by production of the ferryl form of hemoglobin, and also by release of OH− through the Fenton reaction. On the other hand, the other responses to DBBF-Hb injection, such as mast cell degranulation, goblet cell secretion, and eosinophil accumulation, appear to be mediated by the Fenton reaction.
Injection of PS-ODN without DBBF-Hb also caused some cellular damage, although far less than when in the presence of DBBF-Hb. It is possible that this damage was mediated by activation of complement. In monkeys, high concentrations of oligonucleotides have caused activation of the alternative complement pathway [Citation[26]]. The complement system provides the first line of defense against foreign cells or particles, ensuring their phagocytic removals. When complement C3a is formed, it equilibriates rapidly across capillaries due to its low molecular weight (9000). The peak concentration in blood is reached in 15 minutes, and then it rapidly declines to normal in 60 minutes. This time course is similar to that of the intestinal ultrastructural responses to PS-ODN reported in the present study.
In summary, it appears that iron chelators may be useful in reducing mast cell degranulation and subsequent eosinophil migration into the intestinal tissue following bolus injection of DBBF-Hb. Mast cell degranulation is a hallmark of the inflammatory response, and release of mast cell mediators, such as histamine and leukotrienes, can increase microvascular permeability, resulting in edema and in loss of selectivity of the endothelial barrier, compromising the molecular exchange between blood and tissue. In addition, some mast cell mediators recruit eosinophil into the tissue and the eosinophils cause further mast cell degranulation, resulting in a viscous cycle. The observed reduction of mast cell degranulation and eosinophils recruitment by pre-treatment with the iron chelator, PS-ODN, should therefore help to inhibit the inflammatory reaction. However, this study has demonstrated that to maintain epithelial integrity after bolus injection of DBBF-Hb in villi near Peyers' patches, iron chelation is insufficient, and it appears to be necessary to reduce formation of ferrylHb. The villi near Peyers' patches may be more susceptible to epithelial damage than those in other regions because of the presence of large numbers of inflammatory and immune cells, such as macrophages, neutrophils, eosinophils and lymphocytes. These cells produce H2O2 when activated, which converts hemoglobin to its ferryl form, particularly in the presence of PS-ODN. Several direct strategies are emerging aimed at cycling ferryl back to ferric hemes by stimulating a catalase-like activity of hemoproteins using nitroxide [Citation[27]] or the addition of Trolox, a vitamin E analog known for its anti-ferryl activity [Citation[28]]. Alternatively, it may be advantageous to cross-link the ROS scavengers, superoxide dismutase and catalase, to the hemoglobin molecule [Citation[29]].
ACKNOWLEDGEMENTS
We are grateful to BioPharma, Inc., Corvallis, OR, for providing the PS-ODN. This work was supported by National Heart, Lung and Blood Institute Grant HL-53047.
REFERENCES
- Sloan, E.R., Koenigsberg, M., Gens, D., Cipolle, M., Runge, J., Mullory, M., Rodman, G. (1999). Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhage shock: A randomized controlled efficacy trial. JAMA 282: 1857–1864. [PUBMED], [INFOTRIEVE]
- Przybelski, R., Daily, E.K., Kisicki, J.C., Mattia-Goldberg, C., Bounds, M.J., Colburn, W.A. (1996). Pharmacologic profile of diaspirin cross-linked hemoglobin solution. Crit. Care Med. 24: 1993–2000. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Thompson, A., McGarry, A.E., Valeri, C.R., Lieberthal, W. (1994). Stroma-free hemoglobin increases blood pressure and GFR in the hypotensive rat: Role of nitric oxide. Journal of Applied Physiology 77(5): 2348–2354. [PUBMED], [INFOTRIEVE]
- Feola, M., Simioni, J., Dobke, M., Canizaro, P.C. (1988). Complement activation and the toxicity of stroma-free hemoglobin solutions in primates. Circ. Shock 25: 275–290. [PUBMED], [INFOTRIEVE]
- Bolin, R., Smith, D., Moore, G., Boswell, G., DeVenuto, F. (1983). Hematologic effects of hemoglobin solutions in animals. Progress in clinical & Biological Research 122: 117–126.
- Baldwin, A.L. 1997. Blood substitutes and the intestinal microcirculation: extravasation and ultrastructural alterations, in Advances in Blood Substitutes: Industrial Opportunities and Medical Challenges, R.M. Winslow, K.D. Vandergriff, M. Intaglietta, Eds., Birkhauser: Boston, MA, pp. 19–37.
- Baldwin, A. L., Wilson, L.M., Valeski, J.E. (1998). Ultrastructural effects of intravascularly injected polyethylene glycol-hemoglobin in intestinal mucosa. American J. Physiol. 275 (Heart Circ. Physiol. 44), H615–H625.
- Faivre, B., Labaeye, V., Menu, P., Labrude, P., Vigneron, C. (1994). Assessment of dextran 10-benzene-tetracarboxylate-hemoglobin, an oxygen carrier, using guinea pig isolated bowel model. Art. Cells, Blood Subs., and Immob. Biotech. 23(4): 495–504.
- Gutteridge, J.M.C. (1986). Iron promotors of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 201: 291–295. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Everse, J., Hsia, N. (1997). The toxicities of native and modified hemoglobins. Free Radic. Biol. Med. 22: 1075–1099. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hess, J.R., Macdonald, V.W., Brinkley, W.W. (1993). Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J. Appl. Physiol. 74: 1769–1778. [PUBMED], [INFOTRIEVE]
- Cohen, G. 1985. The fenton reaction, in CRC Handbook for Oxygen Radical Research, Robert A. Greenwald, Ed., CRC Press: Boca Raton, FL.
- Paller, M.S. (1988). Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am. J. Physiol. 255: F539–F544. [PUBMED], [INFOTRIEVE]
- Lee, R., Neya, K., Svizzero, T.A., Vlahakes, G.J. (1995). Limitations of the efficacy of hemoglobin based oxygen carrying solutions. J. Appl. Physiol. 79, 236–242. [PUBMED], [INFOTRIEVE]
- Comair, Y.G., Schipper, H.M., Brem, S. (1993). The prevention of oxyhemoglobin-induced endothelial and smooth muscle cytoskeletal injury by deferoxamine. Neurosurgery 32: 58–64. [PUBMED], [INFOTRIEVE]
- Mata, J.E., Bishop, M.R., Tarantolo, S.R., Angel, C.R., Swanson, S.A., Iversen, P.L. (2000). Evidence of enhanced iron excretion during systemic phosphorothioate oligodeoxynucleotide treatment. Clinical Toxicology 38(4): 383–387. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Winterbourn, C.C. (1985). Reactions of superoxide with hemoglobin, in CRC Handbook of Methods for Oxygen Radical Research, Robert A. Greenwald, Ed., CRC Press: Boca Raton, FL, pp. 137–141.
- Milici, A.J., Bankston, P.W. (1982). Fetal and neonatal rat intestinal capillaries: Permeability to carbon, ferritin, hemoglobin, and myoglobin. Am. J. Anat. 165: 165–186. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Henderson, L.M., Chappell, J.B. (1993). Dihydrorhodamine 123: A fluorescent probe for superoxide generation? European Journal of Biochemistry 217(3): 973–980. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Grisham, M.B., Gaginella, T.S., von Ritter, C., Tamai, H., Be, R.M., Granger, D.N. (1990). Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, and epithelial cell viability. Inflammation 14(5): 531–542. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Giulivi, C., Davies, K.J. (1990). A novel antioxidant role for hemoglobin. The comproportionation of ferrylhemoglobin with oxyhemoglobin. J. Biolog. Chem. 265(32): 19453–19460.
- Borish, L., Joseph, B.Z. (1992). Inflammation and the allergic response. Medical Clinics of North America 76(4): 765–787. [PUBMED], [INFOTRIEVE]
- Cashon, R.E., Alayash, A.I. (1995). Reaction of human HbA0 and two cross-linked derivatives with hydrogen peroxide: Differential behavior of the ferryl intermediate. Arch. Biochem. Biophys. 316: 461–469. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Baldwin, A.L., Wiley, E.B. (2002). Selenium reduces hemoglobin-induced damage to intestinal mucosa. Art. Cells, Blood Subs., and Immob. Biotech. 30(1): 1–22. [CSA], [CROSSREF]
- Suzuki, M., Asako, H., Kubes, P., Jennings, S., Grisham, M.B., Granger, D.N. (1991). Neutrophil-derived oxidants promote leukocyte adherence in postcapillary venules. Microvascular Research 42(2): 125–138. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Henry, S.P., Templin, M.V., Gillett, N., Rojka, J., Levin, A.A. (1999). Correlation of toxicity and pharmacokinetic properties of a phosphorothioate oligonucleotide designed to inhibit ICAM-1. Toxicologic Pathology 27(1): 95–100. [PUBMED], [INFOTRIEVE], [CSA]
- Krishna, M.C., Russo, A., Mitchell, J.B., Goldstein, S., Dafni, H., Samuni, A. (1996). Do nitroxide antioxidants act as scavengers of O2− or as SOD mimics? J. Biol. Chem. 271: 26026–26031. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Giulivi, C., Romero, F.J., Cadenas, E. (1992). The interaction of Trolox C, a water-soluble vitamin E analog, with ferrylmyoglobin: Reduction of the oxoferryl moiety. Archives of Biochemistry & Biophysics 299(2): 302–312. [CROSSREF]
- Razak, S., D'Agnillo, F., Chang, T.M.S. (1997). Crosslinked hemoglobin-superoxide dismutase-catalase scavenges free radicals in a rat model of intestinal ischemia-reperfusion injury. Art. Cells, Blood Subs., and Immob. Biotech. 25: 181–192.