Abstract
Phototherapy is commonly used for the treatment of neonatal jaundice. Riboflavin is a photosensitizer that generates singlet oxygen, which promotes bilirubin photodecomposition. Metalloporphyrins are also effective photosensitizers. The effect of a combined dosing regimen of riboflavin and metalloporphyrins was studied, with the aim of increasing the efficiency of the phototherapeutic treatment of hyperbilirubinemia. It was envisaged that riboflavin and the metalloporphyrins, by promoting the photodecomposition of bilirubin, would thereby lead to a reduction of the toxic side effects associated with phototherapy. The results shows that a phototherapeutic treatment, in which riboflavin and metalloporphyrins were co-administered, was effective in reducing Heme Oxygenase activity. However, a comprehensive study of the possible side effects of metalloporphyrin treatment in the wavelength under consideration is essential prior to utilizing these compounds for any clinical applications.
INTRODUCTION
Physiologic jaundice is still being considered a risk factor during the neonatal period. The phenomenon results from the abrupt cessation of bilirubin clearance by the placenta and a transient deficiency in hepatic bilirubin removal. Phototherapy is routinely used for the treatment of neonatal jaundice. Phototherapy causes the photo-oxidation of bilirubin to colorless lower molecular weight propentodyopents and maleimide derivatives and structural and configurational photoisomerization [Citation[1]]. Riboflavin has been reported to be an effective photosensitizer and is capable of generating singlet oxygen. It can therefore, promote bilirubin photodecomposition [Citation[2-3]].
Administration of tin-protoporphyrin and its derivatives is being considered as an alternative therapy for preventing hyperbilirubenemia by inhibiting heme oxygenase, the rate limiting enzyme in heme catabolism, combining the phototherapeutic and chemotherapeutic modalities may have desirable synergistic effects on plasma bilirubin concentration [Citation[4-5]]. Metalloporphyrins also have photosensitizing properties, which can have serious side effects. This is because metalloporphyrins such as SnPP are potent photosensitizers leading to the oxidation of a large number of organic molecules. The destruction of these compounds is known to cause mortality in neonatal rats treated with metalloporphyrins and exposed to visible light [Citation[6-7]].
We have therefore studied the effect of a combined administration of metalloporphyrins and riboflavin on heme and drug metabolism enzymes in the absence and presence of light.
EXPERIMENTAL METHODOLOGY
Experimental chemicals
Riboflavin, Bovine Serum Albumin, Sodium dithionite, NADPH, Hemin, etc., were obtained from Sigma Chemicals Co., St. Louis, USA. All other chemicals were of highest available purity grade. Porphyrins were obtained from Porphyrin Products, Logan, Utah, USA. All other chemicals and reagents were of highest analytical grades available.
Experimental animals
Male wistar rats of weight range 150–200 g from our laboratory colony were used as experimental models in the investigation. Only healthy animals were taken in individual cages with raised wire mesh floors. The animals were kept on fasting for 20 hours but had free access to water. After 20 hours, the animals were divided into four groups with three animals per group.
Phototherapy treatment of excised liver fraction
The excised liver fractions from the experimental rats treated with riboflavin and metalloporphyrins were taken in Ringer's solution and placed in an organ bath fitted with a tubelight. The tubelight was allowed to illuminate the tissue for ten minutes, after which the sample was removed and subjected to the appropriate enzyme assay.
Tissue preparation and isolation of microsomal fraction
The animals were decapitated, and the liver after phototherapy treatment was perfused in situ with 0.9% NaCl (4°C) until fully blanched. The liver of the control and experimental animals was carried out in 0.25 M sucrose solution according to the method of Hogeboom [Citation[8]] and Umbeit et al. [Citation[9]]. A weighed amount of the clean tissue was dipped in (1:3 w/v) of potassium phosphate buffer (0.1 M, pH 7.4) containing sucrose (0.25 M) and homogenized in a Potter Elvehjem type glass homogenizer. All homogenates were centrifuged at 10000 × g for 20 minutes at 4°C. The supernatant was further centrifuged at 105,000 × g for 60 minutes at 4°C to get the microsomal fraction. The microsomal pellet was used as the source of heme oxygenase.
Estimation of δ-amino levulinic acid synthase (δ-ALA)
ALA-synthase activity was assayed colorimetrically by the method of Sassa et al. [Citation[10]].
Estimation of heme oxygenase (HMOX)
HMOX activity was measured using protoheme (heme, Fe-protoporphyrin-IX) as the substrate according to the procedures as described previously by Frydman et al. [Citation[11]].
Estimation of cytochrome P-450 content
Cytochrome P-450 was measured according to the procedure as described previously by Omura et al. [Citation[12]].
RESULTS
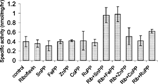
Effect of Administration of Riboflavin and Metalloporphyrins on δ-Amino-Levulinic Acid Synthase (in the Absence of Light)
The administration of riboflavin resulted in a ∼ 68% decline in hepatic δ-ALA-S activity. The administration of FeMP also had a profound effect on hepatic δ-ALA-S levels, diminishing them by almost 61%. The administration of SnMP lowered hepatic ALA-S levels by ∼ 34%. A dosing regimen consisting of ZnPP led to a diminution of ∼ 46% in δ-ALA-S activity.
Figure 1 Effect of riboflavin and metalloporphyrins on ALA-S activity in liver in the absence of light. Adult Wistar rats were administered riboflavin (10 mg/kg bw) and metalloporphyrins.
When a combined dosing regimen of the metalloporphyrin and riboflavin was administered, there was an enhancement of δ-ALA-S activity in most cases. On riboflavin and SnMP administration, there was a remarkable enhancement of hepatic δ-ALA-S levels by ∼ 83%. On administration of ZnPP, however, there was a decline in δ-ALA-S. On the administration of riboflavin and ZnMP there was once again a diminution of δ-ALA-S activity by ∼ 28%.
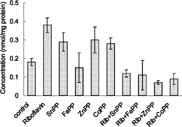
Effect of Administration of Riboflavin and Metalloporphyrins on Heme Oxygenase (in the Absence of Light)
Administration of riboflavin resulted in decline of ∼ 65% in hepatic HMOX levels. A combined dosing regimen of riboflavin and FeMP resulted in a nearly 53% decline of hepatic HMOX levels. The administration of riboflavin and SnPP led to a diminution of about ∼ 46% in HMOX activity.
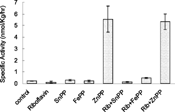
Effect of Riboflavin and Metalloporphyrins on δ-Amino-Levulinic Acid Synthase and Cytochrome P-450 (in the Presence of Light)
When riboflavin was administered and phototherapy was given, a marginal decrease in hepatic ALA-S activity was observed.
SnPP and RuMP administration also resulted in marginal decrease in hepatic ALA activity in the presence of light but a small increase was observed when FePP, ZnPP and CoPP were administered.
Coadministration of riboflavin with metalloporphyrins resulted in an enhancement in hepatic ALA activity in all the cases. ∼ 1 fold increase was observed when SnPP/FePP were administered with riboflavin but a smaller increase was observed on coadministration of riboflavin with ZnPP, CoPP and RuMP.
When riboflavin was administered and subjected to white light, an enhancement of;∼ 62% was observed in hepatic cytochrome P-450 levels. However, administration of SnPP, FePP, ZnPP, CoPP and RuMP showed a rise in cytochrome P-450 levels by ∼ 42%, ∼ 2 folds, ∼ 5 fold, ∼ 1 fold and ∼ 2 fold was observed, respectively.
Figure 4 Effect of riboflavin and metalloporphyrins on cytochrome P-450 in liver in the presence of white light. Adult Wistar rats were administered riboflavin (10 mg/kg bw) and metalloporphyrins.
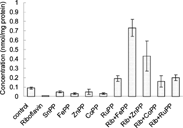
The coadministration of riboflavin with metalloporphyrins resulted in a marked increase in cytochrome P-450 levels in all the cases. Coadministration of SnPP resulted in a rise in cytochrome P-450 levels by ∼ 2 fold, an ∼ 8 fold increase was observed with FePP, ∼ 5 fold with ZnPP and a ∼ 2 fold enhancement was observed with CoPP and RuMP, respectively.
DISCUSSION
Postnatally, bilirubin is normally detoxified and eliminated by enzyme coupling with glucuronic acid and other monosaccharides to give conjugate that are excreted readily in the fetus and early neonate, the enzymic apparatus that conjugates bilirubin is underdeveloped and is inadequate to rapidly metabolize all the bilirubin being produced. Consequently, bilirubin accumulates in some mammals during the first few days of life. This leads to transient hyperbilirubinemia and may result in jaundice [Citation[13-15]]. The most common procedure for treatment is phototherapy. However, phototherapy can have side effects. There is a need to therefore reduce the time of exposure or the intensity of radiation [Citation[16]]. In our study, we have attempted to increase the efficacy of phototherapy by using a photosensitizer riboflavin and synthetic analogs of heme, which inhibit heme oxygenase in vivo, thus decreasing bilirubin formation.
Riboflavin also causes a decline in bilirubin levels by promoting its photocatabolism [Citation[17]]. Various metalloporphyrins were investigated with the aim of identifying specific molecules that could act to enhance the extent of bilirubin decomposition. These included SnPP, FePP, ZnPP, CoPP and RuMP. In all the cases investigated, when riboflavin alone was administered to the rats and the excised liver subjected to white light, an enhancement of ∼ 62% was observed in hepatic cytochrome P-450 levels. This is indicative of a decline in the hepatic heme oxygenase activity. The coadministration of riboflavin with the metalloporphyrins resulted in a marked increase in cytochrome P-450 levels, in all the cases after subjecting the tissue to white light.
Coadministration of SnPP resulted in a rise in the cytochrome P-450 levels by ∼ 2 fold, ∼ 8 fold increase was observed with FePP, ∼ 5 fold with ZnPP and a ∼ 2 fold enhancement was observed with CoPP and RuMP, respectively. Though this methodology proved to be a success in vitro studies, the toxic effects caused due to the photosensitizing properties of all the above metalloporphyrins have been incompletely studied in vivo. However, one may without hesitation define a potential application of riboflavin in enhancing the photodegradation of bilirubin.
REFERENCES
- Ennever, J.F. (1988). Phototherapy for neonatal jaundice. Photochem Photobiol. 47: 871–876. [PUBMED], [INFOTRIEVE], [CSA]
- Edwards, A.M., Silva, E. (2001). Effect of visible light on selected enzymes, vitamins and amino acids. J. Photochem. Photobiol. 63: 126–131. [CSA], [CROSSREF]
- Joshi, P.C. (1989). Ultraviolet radiation-induced photodegradation and 1O2, O2- production by riboflavin, lumichrome and lumiflavin. Indian J. Biochem. Biophys. 26: 186–189. [PUBMED], [INFOTRIEVE], [CSA]
- Tenhunen, R., Marver, H.S., Schmid, R. (1968). The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. 61: 748–755. [PUBMED], [INFOTRIEVE], [CSA]
- Hintz, S.R., Vreman, H.J., Stevenson, D.K. (1990). Mortality of metalloporphyrin-treated neonatal rats after light exposure. Dev. Pharmacol. Ther. 14: 187–192. [PUBMED], [INFOTRIEVE], [CSA]
- Keino, H., Nagae, H., Mimura, S., Watanabe, K., Kashiwamata, S. (1990). Dangerous effects of tin-protoporphyrin plus photoirradiation on neonatal rats. Eur. J. Pediatr. 149: 298–279. [CSA], [CROSSREF]
- Vreman, H.J., Gillman, M.J., Downum, K.R., Stevenson, D.K. (1990). In vitro generation of carbon monoxide from organic molecules and synthetic metalloporphyrins mediated by light. Dev. Pharmacol. Ther. 15: 112–124. [PUBMED], [INFOTRIEVE], [CSA]
- Hogeboom, G.H. (1955). In Methods of Enzymology, Colowick, S.P. and Kaplan, N.O., Eds., Academic Press Inc., NY, p. 16.
- Umbeit, W.W., Burries, R.H., Stauffer, S.F. (1957). Burgess Publishing Co., Minneapolis, 3rd ed.
- Sassa, S., Kappas, A., Bernstein, S.E., Alvares, A.P. (1979). Heme biosynthesis and drug metabolism in mice with hereditary hemolytic anemia. Heme oxygenase induction as an adaptive response for maintaining cytochrome P-450 in chronic hemolysis. J. Biol. Chem. 10: 729–735. [CSA]
- Frydman, R.B., Tomaro, M.L., Buldain, G., Awruch, J., Diaz, L. (1981). Specificity of heme oxygenase: a study with synthetic hemins. Biochemistry, 20: 5177–5182. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Omura, T., Sato, R. (1964). The carbon monoxide-biding pigment of liver microsomes. II. Solubilization, purification and properties. J. Biol. Chem. 239: 2379–2385. [PUBMED], [INFOTRIEVE], [CSA]
- Kawade, N., Onishi, S. (1981). The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem. J. 196: 257–260. [PUBMED], [INFOTRIEVE], [CSA]
- Lightner, D.A., McDonagh, A.F. (2001). Structure and metabolism of natural and synthetic bilirubins. J. Perinatol. S1 3–6. [CSA]
- McDonagh, A.F., Lightner, D.A. (1988). Phototherapy and the photobiology of bilirubin. Semin Liver Dis. 8: 272–283. [PUBMED], [INFOTRIEVE], [CSA]
- Lightner, D.A. (1974). Photochemistry of pyrroles, bile pigments and prophyrins. Photochem. Photobiol. 19: 457–459. [PUBMED], [INFOTRIEVE], [CSA]
- Kostenbauder, H.B., Sanvordeker, D.R. (1973). Riboflavin enhancement of bilirubin photocatabolism in vivo. 29: 282–283. [CSA]