Abstract
In order to demonstrate a new method to label and select enough glial cells from induced MSCs to provide cells for cell therapy, MSCs were induced with Beta-mercaptoethanol followed by retinoic acid, forskolin, basic-FGF, PDGF and heregulin. Induced MSCs were transfected with reconstructed vector pGFAP-EGFP by inserting GFAP promotor into pEGFP-N3 to substitute CMV promotor. Living cells against G418 were enriched and checked by flowcytometry. EGFP expressing cells were sorted and used for transplantation in vivo. Immunoelectronmicroscopy was accomplished using anti-EGFP to relocalize the transplanted cells. Almost all MSCs took on phenotypes of glial cells after induction, expressing S100 and GFAP. The EGFP expression rate of survived MSCs against G418 was 82.74%. Glial cells expressing EGFP accumulated mainly around the damaged nerve fibers. MSCs were relocalized by immunoelectronmicroscopy and remyelination was observed. EGFP expression controlled by GFAP promoter in mesenchymal cells was an efficient tool for glial lineage selection and transplantation. Induced MSCs can promote nerve regeneration by participating remyelination.
INTRODUCTION
Pluripotency and the capacity for continuous self-renewal make mesenchymal cells (MSCs) an attractive donor source for cell-replacement strategies [Citation[1]]. A key prerequisite for a therapeutic application of MSCs is the generation of defined somatic cell populations. Some researchers have induced MSCs into neuroglial and neurons in vitro [Citation[8]]. Dezawa [Citation[5]] and Tohill [Citation[9]] injected MSCs directly to the nerve injure site and verified its abilities to facilitate nerve regeneration. It may be a great advance if a selection procedure in vitro has been established for the enrichment of neuroglial precursor cells from MSCs. Glial fibrillary acid protein (GFAP) is an intermediate filament that is specifically expressed in matured astrocytes. The promoter gene has been cloned successfully [Citation[2]] and has been used in the study of transgenic mice [Citation[3]]. The EGFP expression under the regulation of the GFAP-specific promoter faithfully mimic the endogenous expression of GFAP in transgenic mice. Here, we demonstrated that a targeted insertion of the GFAP promotor to substitute original CMV promotor before EGFP gene in pEGFP-N3 permits efficient fluorescence-activated cell sorting (FACS)-based lineage selection of MSCs-derived glial cells [Citation[10]].
MATERIALS AND METHODS
Reconstruction of Expression Vector
The 2.2kb h-GFAP promoter sequence was dissected from pGfa-LacZ plasmid (gift of Professor Brenner, National Institutes of Health, Betheda, MD) after digested by restricted enzyme Ase I and Sal I (Promaga). The pGFAP-EGFP plasmid was reconstructed by inserting the 2.2kb h-GFAP promoter sequence from pGfa-LacZ plasmid into the sites between the Ase I and Sal I sites of pEGFP-N3 (Clontech BD). The reconstructed plasmid was renamed as pGFAP-EGFP-N3. After being transformed into Escherichia coli DH5α and mass-extraction using Qiagen plasmid mass-extration kit (Qiagen), the reconstructed plasmid was prepared for next transfection procedure.
MSCs Culture
Isolation of adult SD rat MSCs was performed according to the procedure provided by Cuevas [Citation[4]]. The tibias and femurs bone marrow was washed with α-MEM (Gibco) after the rats were killed with overdose pentobarbital (about 100 mg/kg). The perfusate fluid was cultured in α-MEM supplemented with 10% fetal bovine serum and 200 mg/ml Kanamycin. To get rid of the hematic cells, the culture medium was removed by virtue of their adherence character to plastic after 48 hours culture. Leukemia inhibitory factor (LIF) was added to the culture medium to maintain the differentiation abilities [Citation[6]].
Differentiation of MSCs
Subcultured MSCs were incubated in α-MEM supplemented with 10% fetal bovine serum and 1mmol/L β-mercaptoethanol (β-ME) for 24 hours. After the medium was removed, MSCs were washed twice using phosptat buffe solution (PBS). MSCs were cultured with 10% fetal bovine serum and 35 ng/mL all-trans-retinoic acid (RA, Sigma) for 3 days. Subsequently, MSCs were incubated in α-MEM supplemented with 10% fetal bovine serum and mixture containing 5 mmol/L forskolin (FSK, Sigma), 10 ng/mL recombinant human basic-fibroblast growth factor (bFGF, Peprotech, London, UK), 5 ng/mL recombinant human platelet derived growth facor-AA (PDGF-AA, Peprotech, London, UK), and 200 ng/mL recombinant human heregulin-β(HRG-β, R&D System, Minneapolis, MN) for 7 days without any passage [Citation[5]].
Transfection Procedure
The reconstructed plasmid pGFAP-EGFP-N3 was transfected into the induced MSCs using Lipofectamine2000 and Plus Reagent (Invitrogen). Transfection procedure was performed at the time of 90–95% confluence to obtain high efficiency and high expression levels. All procedures were performed according to the transfection instruction. After the fetal bovine serum was added to medium, screening procedure was performed using 400 ug/ml G418 (Clontech) for 10 days.
Immunofluorescence Staining
Induced MSCs were washed in PBS and fixed with 4% formaldehyde in PBS at room temperature for 20 minutes. Immunocytochemistry was performed to discriminate the differentiation characteristics as described using the following primary antibodies against S-100, GFAP (rabbit anti rat, Sigma). Primary antibodies were detected with Rhodamine (TRITC)-conjugated, affinipure labeled, goat anti rabbit IgG(H + L) antibody (Jackson Immunoresearch, US).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Collect Induced MSCs to extract RNA for RT-PCR using TRIzol Reagent (Gibco BRL). The reverse transcription step was carried out in a reaction volume of 25 µl, including reverse transcription enzyme (M-MLV) 200U (Promega); 5 × Buffer 5 µl; d NTP(10 mM)5 µl; Rnasin 20U; Oligo(dt)15 0.5 µg; RNA 1 µl and DEPC water. This reaction volume was kept at 42°C for 60 minutes. After 95°C for 5 minutes to inactivate M-MLV, PCR amplifications were carried out in a reaction volume of 25 µl, including 10 × Buffer 2.5 µl; d NTP(10 mM) 2 µl; Taq E (DNA polymerase) 2U; cDNA 2 µl; Primer pair 12.5 pM respectively and three distilled water. The S100 primer pair was (5-TTGACCTCAACTGAGCAGGA-3) and (5-GCTATGCCCACCTT-CTTCAA-3). The GFAP primer pair was (5-CCAAGCACGCTAATGACT-3) and (5-CTGTAGGTGGCGATCTCGAT-3). (AuGCT Biotechnology Company, China). The GFAP PCR cycling conditions were as follows: 165 seconds at 94°C, 45 seconds at 60°C, 60 seconds at 72°C, and then 35 cycles with 600 seconds for post-extension. The S100 PCR cycling conditions were divided into two steps and were listed as follows: The first step: 160 seconds at 94°C, 45 seconds at 68°C, 60 seconds at 72°C, and then 15 cycles; the second step: 30 seconds at 94°C, 30 seconds at 55°C, 60 seconds at 72°C, and then 20 cycles with 600 seconds for post-extension. The PCR products were collected and 2% agarose gel electrophoresis were performed to discriminate the amplified specific DNA fragment.
Flow Cytometry Analysis of EGFP
The survival cells against G418 were collected for flow cytometric EGFP analyses and preparative green cells sorting, respectively, using FACSort and FACStar Plus sorters (Becton Dickinson, Germany). Cells re-suspended in PBS, at a concentration of 250,000 cells/ml, were analyzed by EGFP fluorescence, with an argon-ion laser operating at 488 nm. Preparative sorting was based on EGFP fluorescence intensity and was performed at flow rates of 1,000–2,000 events/sec. Another un-induced MSCs served as control.
Cell Transplantation and Tissue Analysis
Sprague-Dawley rats (200 g or so, n = 6) were anesthetized with ketamine-HCl and xylazine (80 and 10 mg/kg body weight i.p., respectively). Expose the sciatic nerve. A mechanical force was applied to sciatic nerve (choose right side) and damaged it to the extent that nerve fibers disrupted while epineurium remained continuous with hemostat pincers. The MSCs (0.1 ml in α-MEM, about × 105 cells/inject site) were microinjected into the injured site. As control, 0.1 ml of normal saline solution was injected into the left side.
Three weeks later, animals were perfused transcardially with 4% paraformaldehyde. The nerve segment receiving cell transplantation were removed and cut on a vibratome into 5 µm continuous sections. Selected sections were subjected to fluorescence microscope (Olympus XI 70) to localize the EGFP expressing MSCs cells.
Fixed the selected nerve fragment in glutaric dialdehyde and programmed to immuno-electron microscope (JEOL 100CX-II, Japan) with an antibody to EGFP according to the product instructions. Colloidal gold (Chinese Military Medical Science Institute) was used to be second antibody. The transplanted MSCs were discriminated from other cells by virtue of the distribution of colloidal gold particles and the morphology characteristic of the transplanted MSCs were evaluated.
RESULTS
Vector Reconstruction
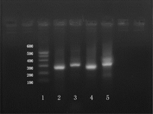
The pGFAP-EGFP plasmid was successfully reconstructed by inserting the 2.2kb h-GFAP promoter sequence from pGfa-LacZ plasmid into the sites between the Ase I and Sal I sites of pEGFP-N3 to substitute the original CMV promoter. The reconstructed vector was about 6.8 kb and the agarose gel electrophoresis confirmation map was shown in .
Figure 1 Limited enzyme incision confirmation map of report vector pGFAP-EGFP-N3. 1 lane: λDNA/Hind III maker, 2 lane: Xho I and Sal I digested fragments, 3 lane: XbalI digested fragments; 4 lane: EcoR I digested fragments. There were three Xbal I limited enzyme incise sites in reconstructed vector and one site was methylated down EGFP, which cannot be digested.
Immunofluorescence Staining
Immunofluorescence staining was carried out to evaluate the nature of induced MSCs and un-induced MSCs using GFAP and S-100, all known as markers of Schwann cells. Our results showed that only induced MSCs were positive for GFAP and S-100, as shown in .
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
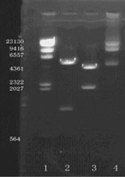
RT-PCR were performed to amplify the DNA sequence expression of GFAP and S100 in induced MSCs. The agarose gel electrophoresis confirmation map was shown in . This result implied that the induced MSCs can transcript the GFAP and S100 sequence at DNA levels.
Flow Cytometry Analysis of EGFP Expression
The survival induced MSCs against G418 were subject to flow cytometry to evaluate the EGFP expression rate. Compared with control group, the EGFP expression rate of induced, transfected and screened MSCs was about 82.74%. This results revealed that the typical analysis map was shown in .
GFAP-Specific EGFP Fluorescence Distribution in Injuried Peripheral Nerve
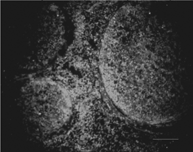
The patterns of distribution of the EGFP-expressing grafted cells were similar in all six experiments. Grafted cells were observed mainly at the site of injury, where the nerve fiber structure had been destroyed and some had come into sites with normal tissue structure. This photo was taken from the proximal nerve segment of damaged nerve site adjacent to the normal nerve site, as shown in .
Immuno-Electron Microscope Morphology Observation
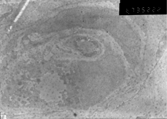
The grafted MSCs can be discriminated from others by expression of colloidal gold particles against anti-EGFP. Three weeks later, the morphology of grafted MSCs was similar to that of Schwann cells. Some MSCs changed their original shape and formed myelinated sheaths, just like Schwann cells, as shown in .
DISCUSSION
Brenner found the gene encoding an intermediate filament protein specific to astrocytes, glial fibrillary acidic protein (GFAP) and identified a GFAP promoter segment that permits transgenes to be expressed specifically in astrocytes in 1994. The GFAP promotor is of interest because it can be turned on as astrocytes mature. The GFAP promoter region derived from pGfa2-cLacz vector span the sequence from − 2163 to + 47, which contains a mutated ATG codon TTG at + 15. We reconstructed the vector pGFAP-EGFP-N3 by inserting the 2.2 kb h-GFAP promoter sequence from pGfa-LacZ plasmid into pEGFP-N3 to substitute the original CMV promoter. The reconstructed vector was confirmed by limited enzyme incision agarose gel electrophoresis. The vector pGFAP-EGFP-N3 was transfected into induced MSCs by lipofectamine 2000 and stable expressing MSCs were screened against G418. About 82.74% of survived MSCs were green under fluorescence microscopy. Almost all induced MSCs were positive to GFAP and S100. The specific GFAP and S100 DNA sequences were successfully amplified and detected by agarose gel electrophoresis. These results revealed that induced MSCs really take on the phenotypes of neuroglial cells, expressing GFAP and S100. The GFAP promoter was active in induced MSCs, triggering specific EGFP expression. Expression of enhanced green fluorescent protein (EGFP) of induced MSCs under the specific-GFAP promoter can serve as a valuable tool to optimize protocols for maintenance and expansion of MSCs in vitro as well as cell tracing in vivo after transplantation.
Three weeks after nerve injury and cells transplantation, the EGFP expressing MSCs mainly accumulated around the injured nerve fiber stem. They extended to almost all the transverse section of the injuried site. To evaluate the MSCs destination and morphyology character, we accomplished immuno-electron microscope using anti-EGFP as primary antibody. From the immuno-electron microscope, we found that EGFP-expressing MSCs survived and their morphyology character was similar to that of Schwann cells of peripherial nerve. The karyons were intact and myelination was found. This results suggested that the induced MSCs can survive and contribute to nerve regeneration by taking on functions of Schwann cells. Although some researchers had contended that cells fusion were the destination of transplanted MSCs in vivo [Citation[7], Citation[11]], the glial cells survived with only one karyons in our immunological electron-microscope study.
Schwann cells are the myelinating glial cells in the peripheral nerve system, and are known to play a key role in Wallerian degeneration and subsequent regeneration process. Activated Schwann cells can irritate a series of reactions, facilitating peripheral nerve regeneration. The treatment target of peripheral nerve defects is aimed at recreating the nerve continuity to allow axonal regrowth into the injuried nerve distal stumps. Some researchers have tried to promote nerve regeneration using artifical nerve conduits filled with autologous Schwann cells. However, the plenitudinous resource of autologous Schwann cells still remains the most difficult problem.
The sequential administration of various factors of BME and RA followed by a mixture of FSK, bFGF, PDGF and HRG effectively induces the differentiation of MSCs into myelinating neuroglial cells (including Schwann cells) which can express the enhanced green fluorescence protein (EGFP) under the GFAP-specific promoter after the transfected with Lipofectamine 2000 transfection kit. In this experiment, MSCs were successfully induced into neuroglial cells, expressing GFAP and S100.
Marrow stromal cells are certainly one of the most attractive cells for an application of neural transplantation because they exhibit several important and potential advantageous features they are easy to isolate from bone marrow aspirates in the clinic and can readily be expanded under culture conditions for autologous transplantation. Therefore, differentiated MSCs are considered to become one of the strongest candidates for cell transplantation involving tissues of the nervous system.
This research was supported by Chinese National 863 Project Grant (2002AA205071) and Chinese National Nature Science Grant (30271306).
We wish to thank Professor Michael Brenner, presently Associate Professor of Neurobiology with a joint appointment in the Department of Physical Medicine & Rehabilitation, Ulabama, USA, for his kindly presentation of pGfa2-cLacZ plasmid including the GFAP-specific promoter sequence and related technical support.
We also wish to thank Professor Mari Dezawa, Department of Anatomy, Yokohama City University School of Medicine, Japan, for his kindly technical support and encouragement.
REFERENCES
- Brazelton, T.R., Rossi, F.M., Keshet, G.I., Blau, H.M. (2000). From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 290: 1775–1779. [PUBMED], [INFOTRIEVE], [CSA]
- Brenner, M. (1994). Structure and transcriptional regulation of the GFAP gene. Brain Pathol. 4(3): 245–57. [PUBMED], [INFOTRIEVE], [CSA]
- Brustle, O., Jones, K.N., Learish, R.D., Karram, K., Choudhary, K., Wiestler, O.D., Duncan, I.D., McKay, R.D. (1999). Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 30;285(5428): 754–6. [CSA]
- Cuevas, P., Carceller, F., Dujovny, M., Garcia-Gomez, I., Cuevas, B., Gonzalez-Corrochano, R., Diaz-Gonzalez, D., Reimers, D. (2002). Peripheral nerve regeneration by bone marrow stromal cells. Neurological Research. 24: 634–638. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Dezawa, M., Takahashi, I., Esaki, M., Takano, M., Sawada, H. (2001). Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. European Journal of Neuroscience. 14: 1771–1776. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jiang, Yuehua, Jahagirdar, Balkrishna, N., Reinhardt, R., Lee, Schwartz, Robert, E., Keene, C., Dirk, Ortiz-Gonzalez, Xilma, R., Reyes, Morayma, Lenvik, Todd, Lund, Troy, Blackstad, Mark, Du, Jingbo, Aldrich, Sara, Lisberg, Aaron, Low, Walter, C., Largaespada, David, A. (2002). Pluripoency of meschymal stem cells derived from adult marrow. Nature. 418: 41–49. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Naohiro Terada, Takashi Hamazaki, Masahiro Oka, Masanori Hoki, Diana M. Mastalerz, Yuka Nakano, Edwin M. Meyer, Laurence Morel, Bryon E. Petersen, Edward W. Scott. (2002). Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 416: 542–545. [CSA], [CROSSREF]
- Sanchez-Ramos, J., Song, S., Cardozo-Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., Freeman, T.B., Saporta, S., Janssen, W., Patel, N., Cooper, D.R., Sanberg, P.R. (2000). Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 164: 247–256. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tohill, M., Mantovani, C., Wiberg, M., Terenghi, G. (2004). Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 27; 362(3): 200–203. [CSA], [CROSSREF]
- Wernig, M., Tucker, K.L., Gornik, V., Schneiders, A., Buschwald, R., Wiestler, O.D., Barde, Y.A., Brustle, O. (2002). Tau EGFP embryonic stem cells: an efficient tool for neuronal lineage selection and transplantation. J Neurosci Res. 15; 69(6): 918–24. [CSA], [CROSSREF]
- Xin Wang, Holger Willenbring, Yassmine Akkari, Yumi Torimaru, Mark Foster, Muhsen Al-Dhalimy, Eric Lagase, Milton Finegold, Susan Olson, Markus Grompe. (2003). Cell fusion is the principal source of bone marrow-derived hepatocytes. Nature. 422: 897–900. [CSA], [CROSSREF]