Abstract
A new urea biosensor was prepared by immobilizing urease with four different procedures on poly(vinylchloride) (PVC) ammonium membrane electrode containing palmitic acid by using nonactine as an ammonium-ionophore. The analytical characteristics were investigated and were compared those of the biosensor prepared by using carboxylated PVC. The effect of pH, buffer concentration, temperature, urease concentration, stirring rate and enzyme immobilization procedures on the response to urea of the enzyme electrode were investigated. The linear working range and sensitivity of the biosensor were also determined. The urea biosensor prepared by using the PVC membranes containing palmitic acid showed more effective performance than those of the carboxylated PVC based biosensors. Additionally, urea assay in serum was successfully carried out by using the standard addition method.
INTRODUCTION
Urea is the final product of human protein metabolism. An important parameter in clinical analysis is urea, where an increased concentration in blood and urine is a strong indicator for renal dysfunction. The determination of urea in body fluids is one of the most frequent analyses in routine clinical laboratories [Citation[5]]. The normal urea range in human serum is between 1.7 and 8.3 mmol/l and levels increase up to 100 mmol/l under pathophysiological conditions [Citation[20]]. Conventional urea sensors based on pH electrodes [Citation[10], Citation[12], Citation[17], Citation[21], Citation[25]] and ammonium-selective electrodes [Citation[3], Citation[5], Citation[8], Citation[9], Citation[15], Citation[26]] have been used to detect the hydrogen ions or ammonium ions produced in the enzymatic reaction.
Urease (E.C.3.5.1.5) is an important enzyme in biological systems. Urea is hydrolized by urease according to the reaction
The major problem for pH-sensitive electrodes is that the sensor response is strongly dependent on the buffer capacity of the sample because the pH change produced in the course of the enzyme-catalyzed reaction is suppressed by the buffer used [Citation[4], Citation[14], Citation[22]]. The general problem of ammonium-sensitive electrodes is interference by Na+-, K+- and Ca+2 ions, which are present in serum (4.5 mmol/l K+ and 140 mmol/l Na+).
The potential of ammonium electrode changed the result of changing of ammonium concentration formed after enzymatic reaction is proportional to urea concentration in serum sample. Therefore, ammonium ions produced during enzymatic urea hydrolysis change the potential of the electrode. This changing is proportional to the urea concentration. Ammonium ions are products of many biocatalytic reactions and an ammonium–selective electrode has been widely used for the construction of different enzymatic sensors for determination of creatinine, adenosine, guanine, AMP, aminoacids, etc. [Citation[11], Citation[17], Citation[17]]. Enzyme electrodes that measure the produced ammonium ions have been investigated in detail and described in the literature [Citation[5], Citation[13-14], Citation[26]]. The best ones were constructed with nonactin as an ionophore and then allowed determination of ammonium ions in a wide range of concentration.
The aim of this work, a new urea biosensor was to develop by using PVC ammonium-selective membrane containing palmitic acid (a long-chain fatty acid) and nonactine as an ammonium ionophore to determine urea in serum.
MATERIALS AND METHODS
Reagents and Apparatus
Urease (EC 3.5.1.5) was obtained from Merck (Darmstadt, FRG). 1-ethyl-3-(3-dimetylaminopropyl)carbodiimide (EDC) was obtained from Sigma Chem. Co. (St. Louis, USA). Nonactin, palmitic acid, bis-(2ethyl)hexyl sebacate (DOS), poly(vinylchloride) (PVC), carboxylated poly(vinylchloride)(PVC-COOH) was obtained from Fluka. All other chemicals used were analytical reagent grade. Standard solutions and buffer solutions were prepared with deionized water.
The blood serum samples were provided by Biochemistry Laboratory at İbn-i Sina Hospital, Ankara University.
Potential and pH measurements were carried out with an ORION 720 model pH-ionmeter.
Preparation of Ammonium-selective Membrane Electrodes
The membrane compositions of ammonium-ion selective electrodes prepared by using PVC containing palmitic acid and carboxylated PVC are shown at .
Table 1. The membrane compositions of ammonium-selective electrodes prepared by using PVC containing palmitic acid and carboxylated PVC
Each membrane according to the compositions at was prepared by dissolving about 400 mg membrane components in 5 ml of tetrahydrofurane (THF). The solutions were poured on a glass plate inside a glass ring (42 mm diameter). After 24 hours drying at room temperature, the membrane was formed. Disks of 5 mm diameter were cut out and attached to the glass electrode body. The inner electrode solution was 10−2 M ammonium chloride (pH 7.0).
Preparation of Urea Biosensors
Enzyme was chemically immobilized on all membrane electrodes surfaces by four different procedures according to the similar procedures given by Koncki et al. [Citation[13]].
A – 30 mg urease and 5 mg carbodiimide were dissolved in 1 ml bidistilled water. 50 µl of this solution was dropped on the membranes surface of ammonium-selective electrodes and left overnight (Biosensor A).
B – 50 µl of 2.5% glutaraldehyde solution was deposited on the surface of electrode A and for 0.5 hours. Then the electrode surface was washed with bidistilled water to remove the excess of glutaraldehyde (Biosensor B).
C – 50 µl of urease suspension prepared in bidistilled water (20 mg/ml) was deposited on the electrode B surface. The electrode was left overnight (Biosensor C).
D – Electrode C was placed in phosphate buffer at pH 7.0 (10 ml) and 20 mg sodium borohydride was added by stirring vigorously. Electrode was left for 1 hour (Biosensor D).
To remove the excess of unbounded enzymes all types of biosensors were left for 1 hour vigorously stirred phosphate buffer (pH 7.0, 10 mM). When not in use, biosensors were stored in a refrigerator at 4°C.
Measurement of the Biosensor Response
Potential measurements were carried out by varying urea concentration in steady-state condition. 5 mM of TRIS buffer was used as a working buffer solution (pH 7.0). The following electrochemical cell was formed with the proposed urea biosensors using a Ag/AgCl reference electrode.
Measurements were made with the proposed urea biosensor and reference electrode immersed to a depth of 1.5 cm in urea solution and the solution stirred by a magnetic stirrer. The pH values were determined using an Orion combinated glass-pH electrode. All the experimental works were carried out at 20 ± 1°C.
The calibration curves were obtained by plotting the potential values of a series of standart urea solution against the logarithm of urea concentration. The selectivity coefficients were evaluated by the separated solution method according to the IUPAC recommendation [Citation[24]].
Procedure for Determination of Urea in Serum
Serum samples were collected from hemodialysis patients. The urea in human serum sample was determined by using the standard addition method. The urea biosensor was immersed in the 5 mM TRIS buffer containing certain amount of serum. Then, stock urea solution was added to this solution. Potential values against urea concentrations were plotted and total ammonium amount was determined. Additionally, ammonium concentration in serum samples was determined with standard addition method by using ammonium-selective electrode prepared by same procedure. Urea concentration in serum was calculated by substracting this value from total ammonium amount [Citation[17]]. In addition Berthelot method [Citation[7]] was used for determining serum urea.
RESULTS AND DISCUSSION
Until now, carboxylated PVC has been used as a membrane matrix for construction of urea biosensors based on ammonium electrode, and urease has been chemically immobilized on this membrane surface [Citation[13], Citation[25]]. At this work, PVC matrix containing palmitic acid was used as a membrane matrix and urease was covalently immobilized on this fatty acid. After the immobilization, four different type biosensors were prepared (A, B, C, D). Although the biosensors A and B had a good sensitivity to ammonium ions, the sensitivity to urea was poor (that is slope was reasonably low). Results related to the biosensors prepared by procedure C and D have been discussed below.
The Sensitivity of Urea Biosensors to Ammonium ions
In order to understand sensitivity of the urea biosensors to ammonium ions, mV measurements were made with each biosensor in a series of ammonium chloride solutions. Then calibration graphs were plotted and linear working ranges for ammonium ion and slopes of electrodes are given in .
Table 2. Slopes and linear working ranges of the urea biosensors for ammonium ion at 20 ± 1°C
It can be seen from the sensitivities of all urea biosensors towards ammonium ion are very good and in the range of 1.0 × 10−1–1.0 × 10−6M concentration. Therefore we have decided that these urea biosensors can be used for urea determination in biological fluids.
The Sensitivity of Urea Biosensors to Urea
A series of urea solutions were prepared to determine the sensitivity of these biosensors towards urea and the pH values of these solutions were adjusted to pH 7.0 with trizma-base. The calibration curves were obtained and the working ranges and the slopes for all biosensors were determined from these curves. The linear range of these sensors extends from 1.0 × 10−5 to 1.0 × 10−2 M and they showed an apparent Nernstian response within this range. The linear range and slope of each biosensor are represented at .
Table 3. Linear working ranges and slopes of the urea biosensors at 20 ± 1°C
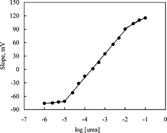
The calibration graph of IC biosensor is shown in as an example. It can be seen from that the sensitivity of the IC electrode prepared by using PVC containing palmitic acid is better than those of the others and the slope of this biosensor was 54.3 ± 0.4 mV/p[urea]. The biosensor was found to give a linear response against ammonium ions in the urea concentration range of 1.0 × 10−2–1.0 × 10−5 M. All biosensors at were found to have a longer working range than those of most of the reported biosensors. The linear ranges of the urea biosensors were found to be to 7.10−2–3.10−4 M by Walcerz et al. [Citation[25]], 10−3–10−5 M by Butt and Camman [Citation[2]] and 2.1 × 10−2–7.2 × 10−5 M by Eggenstein et al. [Citation[5]]. We obtained the working range within 1.0 × 10−2–1.0 × 10−5 M. It can be concluded that the biosensor prepared by using palmitic acid in matrix material is an advantage for urea determination in physiological fluids.
The slopes of the urea biosensors prepared with membranes containing 3% nonactin were bigger than those of the urea biosensors prepared with membranes containing 4% nonactin. Therefore we can conclude that using the membranes containing 3% nonactin was more suitable. These results have shown that the determination of urea can be carried out easily in serum. Our results show that the best procedure for urease immobilization on the membranes investigated for obtaining urea biosensors is procedure C.
Effect of Buffer Concentration
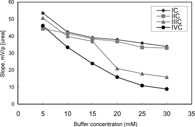
The response of the urea biosensors was measured at six different buffer concentrations varying from 5 mM to 30 mM. The best results for all urea biosensors were obtained at 5 mM TRIS buffer (). The lower buffer concentrations than 5 mM were not investigated due to poor buffering capacity of the solution. Therefore, optimum buffer concentration was accepted 5 mM. Koncki et al. (1994) [Citation[13]] also described that any increase of the buffer concentration caused a decrease of sensitivity. This is in good agreement with our results. A decrease of sensitivies of urea biosensors prepared by using membranes III and IV was bigger than those of membranes I and II as the buffer concentration increased. The slopes of IIIC, IVC biosensors sharply decreased after 15 mM. It can be said that palmitic acid caused to have a good characterization of biosensor.
Effect of Buffer pH
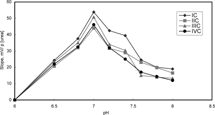
Effect of buffer pH on the analytical signal of the urea biosensors was investigated by measurements in 5 mM TRIS buffer at six different pH in range of 7.0–8.0. It is shown that the slopes and sensitivities of urea biosensors are gradually increased until pH 7.0 and decrease after pH 7.0 (). Above pH 7.0, urease is denaturated due to deprotonation at active site. At pH less than 7.0, urease can also be denaturated and hence reduce enzyme activity [Citation[1]]. The response was also diminished with increase in buffer pH, which is common of the potentiometric systems [Citation[20]]. pH 7.0 was therefore used for all other measurements.
Effect of Temperature
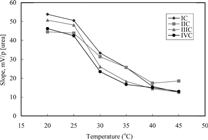
Effect of temperature on analytical signal of these biosensors was investigated by measurements for six different temperatures changing from 20°C to 45°C. As is seen from , the slopes of biosensors remain almost constant between 20 and 25°C and therefore it is thought that urea determinations can be carried out at room temperature by using these sensors. It is shown that the slopes decrease after 25°C because of decrease in the activity of urease. In literature, it was found that the optimum working temperature of the urea biosensor prepared by immobilizing urease with different procedure was also 37°C [Citation[12]].
Selectivity Coefficients of Urea Biosensors
The prepared urea biosensors were tested in view of sensitivity towards sodium, potassium and calcium ions (as chlorides, pH 7.0), and their selectivity coefficients were evaluated by the separated solution method [Citation[23]].
Interference effects of Na+, K+, Ca+2 ions on the response of the urea biosensors were low, but K+ ions showed the most interference among these cations. The results obtained by Walcerz et al. [Citation[25]] and Koncki et al. [Citation[13]] were competitive with our results.
Effect of Stirring Rate
To study the effect of stirring rate on the responses of urea biosensors, the measurements were done in 5 mM TRIS buffer (pH: 7,0) at three different stirring rates (100, 250, and 750 rpm) after reaching stable potential. When stirring was made at 100 and 750 rpm levels, the time to reach stable potential was longer than those of 250 rpm rate level. An increase of stirring rate has resulted in influence on the magnitude of the analytical signal, but caused an increase in the response time of sensors. This situation can be explained in that enzymatic reaction is slow at low stirring rate and probability of enzymatic reaction is decreased because of the changes in the conformation of enzyme at high stirring rate. Therefore 250 rpm is determined by the best stirring rate. Walcerz et al. [Citation[25]], however, indicated that the stirring rate was a negligible effect on the biosensor characteristics.
Response Time
The response time of the urea biosensors was determined by recording the time elapsed to reach a stable potential value after the biosensor and the reference electrode were immersed in calibration solutions. This response time changed between 1–2 minutes, however the response time of IC biosensor was less than 1 minute. These results were shorter than the results obtained for many urea biosensor [Citation[2], Citation[5], Citation[25]]. In conclusion, the response time of the biosensors that we prepared is better than most similar biosensors reported in the literature.
Lifetime of the Urea Biosensors
The lifetimes of all urea biosensors were determined by reading the potential values of the calibration solutions and by plotting the calibration curves for a period of two months. The slope of the biosensors was observed to show a gradual decrease after 60 days. We can therefore conclude that the lifetime of the proposed IC biosensors prepared by the use of palmitic acid is at least two months; however, less than one month for IIIC and IVC prepared by using carboxylated PVC.
Reproducibility of the Urea Biosensors
The biosensors were tested for reproducibility by plotting the calibration curves for five times within one day with same biosensor and the slopes of the biosensors were determined. The relative standard deviation of the slopes was found to be less than 0.5 for IC biosensor and 1.0 % for other seven biosensors.
Application to Urea Determination in Human Serum of the Urea Biosensors
The proposed urea biosensor-based determinations of urea were performed in human serum of hemodialysis patients in a concentration range of 20–120 mg/dl by using the standard addition method. The results were correlated with the Berthelot method employing spectrophotometric indications. There was a good correlation of the urea biosensor method to the Berthelot method (y = 0.966x + 2.0772, R2 = 0.9998, n = 4), and there was no systematic error at 95% confidence level. There were no significant interferences with other substances in human samples which would have reduced the value of the new urea biosensor-based method.
Figure 5 Correlation of urea concentration in human serum (range 10–120 mg/dL) determined by the proposed urea biosensor and by the Berthelot method (y = 0.966x + 2.0772, R2 = 0.9998, n = 4).
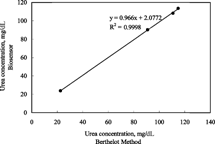
As a result, it can be said that the presented PVC matrix ammonium-selective electrode containing palmitic acid based urea biosensor allowed the determination of urea with comparable accuracy to the Berthelot method in human serum.
CONCLUSION
New types of urea enzyme electrodes based on PVC membrane ammonium-selective electrode containing palmitic acid showed very good analytical parameters: high sensitivity, dynamic stability over 2 months with less decrease of sensitivity, response time 1–2 minutes. It was observed that rapid determinations of serum urea concentrations were also made possible.
We gratefully acknowledge the financial support of T.R. Prime Ministry State Planning Organization (Project No: 98-K-120830).
REFERENCES
- Adeloju, S.B., Shaw, S.J., Wallace, G.G. (1996). Polypyrrole-based amperometric flow injection biosensor for urea. Anal. Chim. Acta. 323: 107–113. [CSA]
- Butt, S.B., Camman, K. (1992). Enzyme urea biosensor based on a modified potentiometric PVC-nonactin membrane electrode for assay of urea in blood. Anal. Lett. 25(9): 1597–1615. [CSA]
- Campanella, L., Mazzei, F., Sammartino, M.P., Tomassetti, M. (1990). New enzyma sensors for urea and creatinine analysis. Bioelectrochem. Bioenergetics. 23: 195–202. [CSA], [CROSSREF]
- Eddowes, M.J. (1987). Response of an enzyme-modified pH-sensitive ion-selective device: analytical solution for the response in the presence of pH buffer. Sensors and Actuators. 11: 265–274. [CSA], [CROSSREF]
- Eggenstein, C., Borchardt, M., Diekmann, C., Gründig, B., Dumschat, C., Cammann, K., Knoll, M., Spener, F. (1999). A disposable biosensor for urea determination in blood based on an ammonium-sensitive transducer. Biosens. Bioelectron. 14: 33–41. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Eggestein, C., Borchardt, M., Dumschat, C., Gründig, B., Cammann, K., Spener, F., Knoll, M. (1995). Potentiometric biosensor in double matrix membrane technology. Biosens. Bioelectron. 10: 595–600. [CSA], [CROSSREF]
- Fawcett, F.K., Scott, J.E. (1960). A rapid and precise method for the determination of urea. J. Clin. Pathol. 13: 156–160. [PUBMED], [INFOTRIEVE], [CSA]
- Guilbault, G.G., Montalvo, J.G. (1969). Urea-spesific enzyme electrode. J. Am. Chem. Soc. 91: 2164–5. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Guilbault, G.G., Montalvo, J.G. (1970). An enzyme electrode for the substrate urea. J. Am. Chem. Soc. 92: 2533–8. [CSA], [CROSSREF]
- Hamann, H., Kühn, M., Böttcher, N., Scheller, F. (1986). Enzyme sensor system for potentiometric urea determination in serum and milk. J. Electroanal. Chem. 209: 69–79. [CSA], [CROSSREF]
- Jasnowska, J., Ligaj, M., Stupnicka, B., Filipiak, M. (2004). DNA sensor for o-dianisidine. Bioelectrochem. 64: 85–90. [CSA], [CROSSREF]
- Karube, I., Tamiya, E., Dicks, J.M. (1986). A microsensor for urea based on an ion-selective field effect transistor. Anal. Chim. Acta. 185: 195–200. [CSA], [CROSSREF]
- Koncki, R., Kopczewska, E., Glab, S. (1994). Comparison of pH-membrane enzyme electrode for urea with covalently bound enzyme. Anal. Lett. 27(3): 475–486. [CSA]
- Koncki, R., Leszcynski, P., Hulanicki, A., Glab, S. (1992). Urea sensor based on glass pH electrodes with physically immobilized urease. Anal. Chim. Acta. 257: 67–72. [CSA], [CROSSREF]
- Koncki, R., Walcerz, I., Ruckruh, F., Glab, S. (1996). Bienzymatic potantiometric electrodes for creatine and L-arginine determination. Anal. Chim. Acta. 333: 215–222. [CSA], [CROSSREF]
- Koncki, R., Chudvick, A., Walcerz, I. (1999). Urea determination using pH-enzyme electrode. J. Pharm. Biomed. Anal., 21: 51–57. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Korenbrot, J.I., Perry, R., Copenhagen, D.R. (1987). Development and characterization of a polymer gel with an immobilized enzyme to measure L-Glutamate. Anal. Biochem. 161: 187–99. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Magalhaes, J.M.C.S., Machado, A.A.S.C. (1998). Urea potentiometric biosensor based on urease immobilized on chitosan membreanes. Talanta. 47: 183–191. [CSA], [CROSSREF]
- Miyahara, Y., Moriizumi, T., Ichimura, K. (1985). Integrated enzyme FETs for simultaneous determination of urea and glucose. Sensors Actuators 7: 1–10. [CSA], [CROSSREF]
- Rick, W. (1990). Klinishe Chemie und Mikroskopie. Springer-Verlag; Berlin, 245–7.
- Ru-Qin, Y., Zong-Rang, Z., Guo-Li, S. (2000). Potentiometric sensors: aspects of the recent development. Sensors Actuators B. 65: 150–153. [CSA], [CROSSREF]
- Ruzicka, J., Hansen, E.H., Ghose, A.K., Mottola, H.A. (1979). Enzyme determination of urea in serum based on pH measurement with the flow injection method. Anal. Chem. 51: 199–203. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Senillou, A., Jaffrezic-Renault, N., Martelet, C., Cosnier, S. (1999). A miniaturized urea based on the integration of both ammonium based urea enzyme field effect transistor and a reference field effect transistor in a single chip. Talanta. 50: 219–226. [CSA], [CROSSREF]
- Schneider, J., Gr. Umezawa, Y., Bühlmann, P., Umezawa, K., Tohda, K., Amemiya, S. (2000). Pure Appl. Chem. 72(10): 1851–2082. [CSA]
- Vadgama, P.M., Alberti, K.G.M.M., Covington, A.K. (1982). Determination of urea in blood plasma by enzyme-pH electrode. Anal. Chim. Acta. 136: 403–6. [CSA], [CROSSREF]
- Walcerz, I., Koncki, R., Leszczynska, E., Salamonowicz, B., Glab, S. (1996). Enzyme biosensors for urea determination based on an ionophore free membrane electrode. Anal. Lett. 29(11): 1939–1953. [CSA]